Anti-integrin αvβ6 autoantibodies are a potential biomarker for ulcerative colitis-like immune checkpoint inhibitor-induced colitis
- PMID: 38461170
- PMCID: PMC11058246
- DOI: 10.1038/s41416-024-02647-1
Anti-integrin αvβ6 autoantibodies are a potential biomarker for ulcerative colitis-like immune checkpoint inhibitor-induced colitis
Abstract
Background: No specific biomarker for immune checkpoint inhibitor (ICI)-induced colitis has been established. Previously, we identified anti-integrin αvβ6 autoantibodies in >90% of patients with ulcerative colitis (UC). Given that a subset of ICI-induced colitis is similar to UC, we aimed to clarify the relationship between such autoantibodies and ICI-induced colitis.
Methods: Serum anti-integrin αvβ6 autoantibody levels were compared between 26 patients with ICI-induced colitis and 157 controls. Endoscopic images of ICI-induced colitis were centrally reviewed. Characteristics of anti-integrin αvβ6 autoantibodies in the ICI-induced colitis patients were compared with those of UC patients.
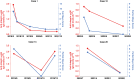
Results: Anti-integrin αvβ6 autoantibodies were found in 8/26 (30.8%) patients with ICI-induced colitis and 3/157 (1.9%) controls (P < 0.001). Patients with anti-integrin αvβ6 autoantibodies had significantly more typical UC endoscopic features than those without the autoantibodies (P < 0.001). Anti-integrin αvβ6 autoantibodies in ICI-induced colitis patients were associated with grade ≥3 colitis (P = 0.001) and steroid resistance (P = 0.005). Anti-integrin αvβ6 autoantibody titers correlated with ICI-induced colitis disease activity. Anti-integrin αvβ6 autoantibodies of ICI-induced colitis exhibited similar characteristics to those of UC.
Conclusions: Anti-integrin αvβ6 autoantibodies may serve as potential biomarkers for the diagnosis, classification, risk management, and monitoring the disease activity, of ICI-induced colitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures