Identification of the growth cone as a probe and driver of neuronal migration in the injured brain
- PMID: 38461182
- PMCID: PMC10924819
- DOI: 10.1038/s41467-024-45825-8
Identification of the growth cone as a probe and driver of neuronal migration in the injured brain
Abstract
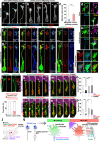
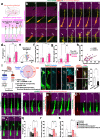
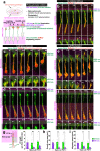
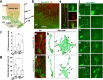
Axonal growth cones mediate axonal guidance and growth regulation. We show that migrating neurons in mice possess a growth cone at the tip of their leading process, similar to that of axons, in terms of the cytoskeletal dynamics and functional responsivity through protein tyrosine phosphatase receptor type sigma (PTPσ). Migrating-neuron growth cones respond to chondroitin sulfate (CS) through PTPσ and collapse, which leads to inhibition of neuronal migration. In the presence of CS, the growth cones can revert to their extended morphology when their leading filopodia interact with heparan sulfate (HS), thus re-enabling neuronal migration. Implantation of an HS-containing biomaterial in the CS-rich injured cortex promotes the extension of the growth cone and improve the migration and regeneration of neurons, thereby enabling functional recovery. Thus, the growth cone of migrating neurons is responsive to extracellular environments and acts as a primary regulator of neuronal migration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Glypican-2 defines age-dependent axonal response to chondroitin sulfate.Exp Neurol. 2023 Aug;366:114444. doi: 10.1016/j.expneurol.2023.114444. Epub 2023 May 15. Exp Neurol. 2023. PMID: 37196696
-
Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension.Science. 2011 Apr 22;332(6028):484-8. doi: 10.1126/science.1200840. Epub 2011 Mar 31. Science. 2011. PMID: 21454754 Free PMC article.
-
The Difference in the Cytoskeletal Machinery of Growth Cones of Growing Axons and Leading Processes.Dev Neurosci. 2022;44(6):455-465. doi: 10.1159/000522200. Epub 2022 Jan 31. Dev Neurosci. 2022. PMID: 35100584
-
Drebrin in Neuronal Migration and Axonal Growth.Adv Exp Med Biol. 2017;1006:141-155. doi: 10.1007/978-4-431-56550-5_9. Adv Exp Med Biol. 2017. PMID: 28865019 Review.
-
Protein tyrosine phosphatase σ in proteoglycan-mediated neural regeneration regulation.Mol Neurobiol. 2013 Feb;47(1):220-7. doi: 10.1007/s12035-012-8346-x. Epub 2012 Sep 7. Mol Neurobiol. 2013. PMID: 22956273 Review.
Cited by
-
Neuraminidase inhibition promotes the collective migration of neurons and recovery of brain function.EMBO Mol Med. 2024 Jun;16(6):1228-1253. doi: 10.1038/s44321-024-00073-7. Epub 2024 May 24. EMBO Mol Med. 2024. PMID: 38789599 Free PMC article.
-
High spatial resolution gene expression profiling and characterization of neuroblasts migrating in the peri-injured cortex using photo-isolation chemistry.Front Neurosci. 2025 Jan 7;18:1504047. doi: 10.3389/fnins.2024.1504047. eCollection 2024. Front Neurosci. 2025. PMID: 39840011 Free PMC article.
-
Neuronal guidance behaviours: the primary cilium perspective.Front Cell Dev Biol. 2025 Jun 30;13:1612555. doi: 10.3389/fcell.2025.1612555. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40661145 Free PMC article. Review.
-
Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons.Bio Protoc. 2025 Mar 20;15(6):e5251. doi: 10.21769/BioProtoc.5251. eCollection 2025 Mar 20. Bio Protoc. 2025. PMID: 40395842 Free PMC article.
-
A mathematical model suggests collectivity and inconstancy enhance the efficiency of neuronal migration in the adult brain.PLoS Comput Biol. 2025 Jun 5;21(6):e1013105. doi: 10.1371/journal.pcbi.1013105. eCollection 2025 Jun. PLoS Comput Biol. 2025. PMID: 40471910 Free PMC article.
References
-
- Cajal SR. A quelle époque apparaissent les expansions des cellules nerveuses de la moelle épinière du poulet. Anat. Anz. 1890;5:609–613.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

