mTOR inhibitor reduces nontumour-related death in liver transplantation for hepatocellular carcinoma
- PMID: 38461206
- PMCID: PMC10924815
- DOI: 10.1186/s43556-024-00170-6
mTOR inhibitor reduces nontumour-related death in liver transplantation for hepatocellular carcinoma
Abstract
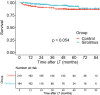
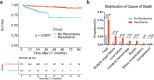
Sirolimus is a regularly applied immunosuppressant for patients undergoing liver transplantation (LT) for hepatocellular carcinoma (HCC). Sirolimus not only significantly inhibits HCC recurrence but also protects renal function. However, the improvement effect of sirolimus on nontumour-related death in patients is still unknown. The aim of our study was to investigate the therapeutic effect of sirolimus on nontumour-related deaths. In this study, we retrospectively enrolled 403 LT patients with HCC from January 1, 2015, to December 31, 2018. The median follow-up time was 47.1 months. The patients were divided into the sirolimus group (N = 184) and the sirolimus-free group (N = 219). There were no significant differences between the sirolimus group and the sirolimus-free group in survival (P = 0.054). In transplant patients who exceeded the Milan or Hangzhou criteria, the sirolimus group achieved higher survival than the sirolimus-free group (P = 0.005; P = 0.02). Moreover, multivariate analysis showed that sirolimus strongly reduced the hazard ratio (HR) for nontumour-related death in LT patients who exceeded the Milan (HR: 0.42; 95% CI: 0.18-1; P = 0.05) or Hangzhou criteria (HR: 0.26; 95% CI: 0.08-0.89; P = 0.032). HCC recurrence increased the risk of nontumour-related death. In conclusion, sirolimus-based immunosuppression can significantly reduce nontumour-related death in LT patients who exceed the criteria for transplantation. In addition, this finding will further promote the application of sirolimus after liver transplantation for hepatocellular carcinoma.
Keywords: Hepatocellular carcinoma; Liver transplantation; Nontumour-related death; Sirolimus.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFA1100500/National Key Research and Development Program of China
- 92159202/National Natural Science Foundation of China
- 82273270/National Natural Science Foundation of China
- 32171368/National Natural Science Foundation of China
- 2017ZX10203205/Major Program of National Fund of Philosophy and Social Science of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
