Bacteriophage therapy for the treatment of Mycobacterium tuberculosis infections in humanized mice
- PMID: 38461214
- PMCID: PMC10924958
- DOI: 10.1038/s42003-024-06006-x
Bacteriophage therapy for the treatment of Mycobacterium tuberculosis infections in humanized mice
Abstract
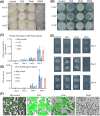
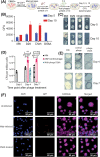
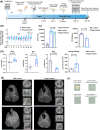
The continuing emergence of new strains of antibiotic-resistant bacteria has renewed interest in phage therapy; however, there has been limited progress in applying phage therapy to multi-drug resistant Mycobacterium tuberculosis (Mtb) infections. In this study, we show that bacteriophage strains D29 and DS6A can efficiently lyse Mtb H37Rv in 7H10 agar plates. However, only phage DS6A efficiently kills H37Rv in liquid culture and in Mtb-infected human primary macrophages. We further show in subsequent experiments that, after the humanized mice were infected with aerosolized H37Rv, then treated with DS6A intravenously, the DS6A treated mice showed increased body weight and improved pulmonary function relative to control mice. Furthermore, DS6A reduces Mtb load in mouse organs with greater efficacy in the spleen. These results demonstrate the feasibility of developing phage therapy as an effective therapeutic against Mtb infection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Bacteriophage therapy for the treatment of Mycobacterium tuberculosis infections in humanized mice.bioRxiv [Preprint]. 2023 Feb 21:2023.01.23.525188. doi: 10.1101/2023.01.23.525188. bioRxiv. 2023. Update in: Commun Biol. 2024 Mar 9;7(1):294. doi: 10.1038/s42003-024-06006-x. PMID: 36747734 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

