Label-free functional analysis of root-associated microbes with dynamic quantitative oblique back-illumination microscopy
- PMID: 38461279
- PMCID: PMC10925023
- DOI: 10.1038/s41598-024-56443-1
Label-free functional analysis of root-associated microbes with dynamic quantitative oblique back-illumination microscopy
Abstract
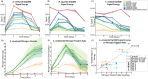
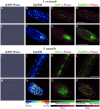
The increasing global demand for food, coupled with concerns about the environmental impact of synthetic fertilizers, underscores the urgency of developing sustainable agricultural practices. Nitrogen-fixing bacteria, known as diazotrophs, offer a potential solution by converting atmospheric nitrogen into bioavailable forms, reducing the reliance on synthetic fertilizers. However, a deeper understanding of their interactions with plants and other microbes is needed. In this study, we introduce a recently developed label-free 3D quantitative phase imaging technology called dynamic quantitative oblique back-illumination microscopy (DqOBM) to assess the functional dynamic activity of diazotrophs in vitro and in situ. Our experiments involved three different diazotrophs (Sinorhizobium meliloti, Azotobacter vinelandii, and Rahnella aquatilis) cultured on media with amendments of carbon and nitrogen sources. Over 5 days, we observed increased dynamics in nutrient-amended media. These results suggest that the observed bacterial dynamics correlate with their metabolic activity. Furthermore, we applied qOBM to visualize microbial dynamics within the root cap and elongation zone of Arabidopsis thaliana primary roots. This allowed us to identify distinct areas of microbial infiltration in plant roots without the need for fluorescent markers. Our findings demonstrate that DqOBM can effectively characterize microbial dynamics and provide insights into plant-microbe interactions in situ, offering a valuable tool for advancing our understanding of sustainable agriculture.
Keywords: Label-free imaging; Nitrogen fixation; Quantitative phase imaging.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Update of
-
Label-Free Functional Analysis of Root-Associated Microbes with Dynamic Quantitative Oblique Back-illumination Microscopy.Res Sq [Preprint]. 2023 Nov 2:rs.3.rs-3517586. doi: 10.21203/rs.3.rs-3517586/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 9;14(1):5812. doi: 10.1038/s41598-024-56443-1. PMID: 37961396 Free PMC article. Updated. Preprint.
Similar articles
-
Label-Free Functional Analysis of Root-Associated Microbes with Dynamic Quantitative Oblique Back-illumination Microscopy.Res Sq [Preprint]. 2023 Nov 2:rs.3.rs-3517586. doi: 10.21203/rs.3.rs-3517586/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 9;14(1):5812. doi: 10.1038/s41598-024-56443-1. PMID: 37961396 Free PMC article. Updated. Preprint.
-
Functional imaging with dynamic quantitative oblique back-illumination microscopy.J Biomed Opt. 2022 Jun;27(6):066502. doi: 10.1117/1.JBO.27.6.066502. J Biomed Opt. 2022. PMID: 35773755 Free PMC article.
-
Metabolic Model of the Nitrogen-Fixing Obligate Aerobe Azotobacter vinelandii Predicts Its Adaptation to Oxygen Concentration and Metal Availability.mBio. 2021 Dec 21;12(6):e0259321. doi: 10.1128/mBio.02593-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903060 Free PMC article.
-
Liberation of amino acids by heterotrophic nitrogen fixing bacteria.Amino Acids. 2005 Jun;28(4):363-7. doi: 10.1007/s00726-005-0178-9. Epub 2005 Apr 15. Amino Acids. 2005. PMID: 15827688 Review.
-
Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture.Microbiol Res. 2018 Jan;206:131-140. doi: 10.1016/j.micres.2017.08.016. Epub 2017 Oct 17. Microbiol Res. 2018. PMID: 29146250 Review.
Cited by
-
Acetic acid enabled nuclear contrast enhancement in epi-mode quantitative phase imaging.J Biomed Opt. 2025 Feb;30(2):026501. doi: 10.1117/1.JBO.30.2.026501. Epub 2025 Feb 4. J Biomed Opt. 2025. PMID: 39906483 Free PMC article.
References
-
- Doty, S. L. Functional Importance of the Plant Endophytic Microbiome: Implications for Agriculture, Forestry, and Bioenergy (Springer, 2017).
-
- Singh, B. & Ryan, J. Managing Fertilizers to Enhance Soil Health 1–24 (International Fertilizer Industry Association, 2015). 10.1364/BOE.416731.
-
- Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M. & Glick, B. R. Plant growth-promoting bacterial endophytes. Microbiol. Res.183, 92–99. 10.1016/j.micres.2015.11.008 (2016). - PubMed
-
- Ahemad, M. & Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci.26, 1–20. 10.1016/j.jksus.2013.05.001 (2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

