Histological, immunohistochemical and serological investigations of the ovary during follicular phase of estrous cycle in Saidi sheep
- PMID: 38461282
- PMCID: PMC10924360
- DOI: 10.1186/s12917-024-03933-z
Histological, immunohistochemical and serological investigations of the ovary during follicular phase of estrous cycle in Saidi sheep
Abstract
Background: Saidi sheep are the most abundant ruminant livestock species in Upper Egypt, especially in the Assiut governorate. Sheep are one of the most abundant animals raised for food in Egypt. They can convert low-quality roughages into meat and milk in addition to producing fiber and hides therefore; great opportunity exists to enhance their reproduction. Saidi breed is poorly known in terms of reproduction. So this work was done to give more information on some hormonal, oxidative, and blood metabolites parameters in addition to histological, histochemical and immunohistochemical investigations of the ovary during follicular phase of estrous cycle. The present study was conducted on 25 healthy Saidi ewes for serum analysis and 10 healthy ewes for histological assessment aged 2 to 5 years and weighted (38.5 ± 2.03 kg).
Results: The follicular phase of estrous cycle in Saidi sheep was characterized by the presence of ovarian follicles in different stages of development and atresia in addition to regressed corpus luteum. Interestingly, apoptosis and tissue oxidative markers play a crucial role in follicular and corpus luteum regression. The most prominent features of the follicular phase were the presence of mature antral (Graafian) and preovulatory follicles as well as increased level of some blood metabolites and oxidative markers. Here we give a new schematic sequence of ovarian follicles in Saidi sheep and describing the features of different types. We also clarified that these histological pictures of the ovary was influenced by hormonal, oxidative and blood metabolites factors that characterizes the follicular phase of estrous cycle in Saidi sheep.
Conclusion: This work helps to understanding the reproduction in Saidi sheep which assist in improving the reproductive outcome of this breed of sheep. These findings are increasingly important for implementation of a genetic improvement program and utilizing the advanced reproductive techniques as estrous synchronization, artificial insemination and embryo transfer.
Keywords: Antioxidants; Estrous cycle; Ovarian follicles; Ovary; PRA; SOD2; Saidi sheep.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


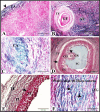

 ) theca externa (arrow) of growing (GF) and antral follicles (AF). Note the orcein positive granulosa cells (G) of antral follicles and the orcein negative zona pellucida (Z) and the cytoplasm of the oocyte. Original magnification; A, B & D: X40, scale bar = 500 µm, C & E: X100, scale bar = 200 µm, F: X 400, scale bar = 50 µm, A-C: Picro-Sirius red technique and D-F: Orcien stain
) theca externa (arrow) of growing (GF) and antral follicles (AF). Note the orcein positive granulosa cells (G) of antral follicles and the orcein negative zona pellucida (Z) and the cytoplasm of the oocyte. Original magnification; A, B & D: X40, scale bar = 500 µm, C & E: X100, scale bar = 200 µm, F: X 400, scale bar = 50 µm, A-C: Picro-Sirius red technique and D-F: Orcien stain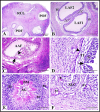




Similar articles
-
Uterine histomorphological and immunohistochemical investigation during the follicular phase of estrous cycle in Saidi sheep.BMC Vet Res. 2025 Jan 13;21(1):16. doi: 10.1186/s12917-024-04456-3. BMC Vet Res. 2025. PMID: 39806411 Free PMC article.
-
LH pulse frequency and the emergence and growth of ovarian antral follicular waves in the ewe during the luteal phase of the estrous cycle.Reprod Biol Endocrinol. 2009 Jul 28;7:78. doi: 10.1186/1477-7827-7-78. Reprod Biol Endocrinol. 2009. PMID: 19638235 Free PMC article.
-
Distribution of visible follicles on the ovarian surface in ewes.J Anim Sci. 1982 Jun;54(6):1196-20. doi: 10.2527/jas1982.5461196x. J Anim Sci. 1982. PMID: 7201996
-
The use of prostaglandins in controlling estrous cycle of the ewe: a review.Theriogenology. 2013 Feb;79(3):399-408. doi: 10.1016/j.theriogenology.2012.10.022. Epub 2012 Dec 6. Theriogenology. 2013. PMID: 23219520 Review.
-
Reproductive cycles in sheep.Anim Reprod Sci. 2011 Apr;124(3-4):259-68. doi: 10.1016/j.anireprosci.2011.02.024. Epub 2011 Feb 23. Anim Reprod Sci. 2011. PMID: 21411253 Review.
Cited by
-
Uterine histomorphological and immunohistochemical investigation during the follicular phase of estrous cycle in Saidi sheep.BMC Vet Res. 2025 Jan 13;21(1):16. doi: 10.1186/s12917-024-04456-3. BMC Vet Res. 2025. PMID: 39806411 Free PMC article.
-
The effect of Norethisterone acetate on the uterus of albino rats: histological, histochemical and ultrastructure study.BMC Vet Res. 2024 Aug 29;20(1):384. doi: 10.1186/s12917-024-04219-0. BMC Vet Res. 2024. PMID: 39210341 Free PMC article.
References
-
- El-Homosi FF, Abd El-Hafiz GA. Reproductive perpormance of Ossimi and Saidi sheep under two pre pubertal planes of nutrition. Assiut Vet Med J. 1982;10:59–66.
-
- Teleb D Teleb D, Ahmed N, El –Din TH, Abou El Soud S, Hassan OM. STUDY ON LEVELS OF SOME BLOOD HORMONAL AND BIOCHEMICAL CONSTITUENTS DURING DIFFERENT REPRODUCTIVE STATUS IN SAIDI EWES. Egypt J Sheep Goats Sci. 2019;9:1–10.
-
- Teleb D, El-Sayed E, Sallam A.E.A. Characterizing the estrous and ovarian activity of Saidi sheep throughout the year. Egypt J Basic Appl Physiol. 2008;7(2):325–39.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

