Deficiency of skeletal muscle Agrin contributes to the pathogenesis of age-related sarcopenia in mice
- PMID: 38461287
- PMCID: PMC10925061
- DOI: 10.1038/s41419-024-06581-1
Deficiency of skeletal muscle Agrin contributes to the pathogenesis of age-related sarcopenia in mice
Abstract
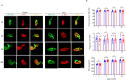
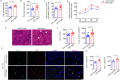
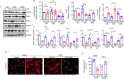
Sarcopenia, a progressive and prevalent neuromuscular disorder, is characterized by age-related muscle wasting and weakening. Despite its widespread occurrence, the molecular underpinnings of this disease remain poorly understood. Herein, we report that levels of Agrin, an extracellular matrix (ECM) protein critical for neuromuscular formation, were decreased with age in the skeletal muscles of mice. The conditional loss of Agrin in myogenic progenitors and satellite cells (SCs) (Pax7 Cre:: Agrin flox/flox) causes premature muscle aging, manifesting a distinct sarcopenic phenotype in mice. Conversely, the elevation of a miniaturized form of Agrin in skeletal muscle through adenovirus-mediated gene transfer induces enhanced muscle capacity in aged mice. Mechanistic investigations suggest that Agrin-mediated improvement in muscle function occurs through the stimulation of Yap signaling and the concurrent upregulation of dystroglycan expression. Collectively, our findings underscore the pivotal role of Agrin in the aging process of skeletal muscles and propose Agrin as a potential therapeutic target for addressing sarcopenia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- De Spiegeleer A, Beckwee D, Bautmans I, Petrovic M, Sarcopenia Guidelines Development group of the Belgian Society of G, Geriatrics. Pharmacological Interventions to Improve Muscle Mass, Muscle Strength and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-analyses. Drugs Aging. 2018;35:719–34. doi: 10.1007/s40266-018-0566-y. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82260287/National Natural Science Foundation of China (National Science Foundation of China)
- 32260194/National Natural Science Foundation of China (National Science Foundation of China)
- 32222034/National Natural Science Foundation of China (National Science Foundation of China)
- 20224ACB216006/Natural Science Foundation of Jiangxi Province (Jiangxi Province Natural Science Foundation)
- 20224BAB206038/Natural Science Foundation of Jiangxi Province (Jiangxi Province Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

