Neoadjuvant-adjuvant pertuzumab in HER2-positive early breast cancer: final analysis of the randomized phase III PEONY trial
- PMID: 38461323
- PMCID: PMC10925021
- DOI: 10.1038/s41467-024-45591-7
Neoadjuvant-adjuvant pertuzumab in HER2-positive early breast cancer: final analysis of the randomized phase III PEONY trial
Abstract
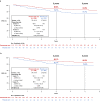
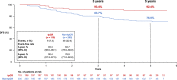
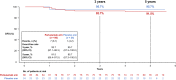
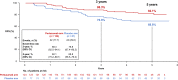
The randomized, multicenter, double-blind, placebo-controlled, phase III PEONY trial (NCT02586025) demonstrated significantly improved total pathologic complete response (primary endpoint) with dual HER2 blockade in HER2-positive early/locally advanced breast cancer, as previously reported. Here, we present the final, long-term efficacy (secondary endpoints: event-free survival, disease-free survival, overall survival) and safety analysis (62.9 months' median follow-up). Patients (female; n = 329; randomized 2:1) received neoadjuvant pertuzumab/placebo with trastuzumab and docetaxel, followed by adjuvant 5-fluorouracil, epirubicin, and cyclophosphamide, then pertuzumab/placebo with trastuzumab until disease recurrence or unacceptable toxicity, for up to 1 year. Five-year event-free survival estimates are 84.8% with pertuzumab and 73.7% with placebo (hazard ratio 0.53; 95% confidence interval 0.32-0.89); 5-year disease-free survival rates are 86.0% and 75.0%, respectively (hazard ratio 0.52; 95% confidence interval 0.30-0.88). Safety data are consistent with the known pertuzumab safety profile and generally comparable between arms, except for diarrhea. Limitations include the lack of ado-trastuzumab emtansine as an option for patients with residual disease and the descriptive nature of the secondary, long-term efficacy endpoints. PEONY confirms the positive benefit:risk ratio of neoadjuvant/adjuvant pertuzumab, trastuzumab, and docetaxel treatment in this patient population.
© 2024. The Author(s).
Conflict of interest statement
All authors report receiving research support in the form of third-party writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd. L.H., D.P., H.Y., W.L., S.W., S.C., N.L., Y.W., C.W., Y-C.C., H-C.W., S.Y.K., J.H.S., K.S., Z.J., and Z.S. report contracted research from F. Hoffmann-La Roche Ltd. S.L. reports contracted research from F. Hoffmann-La Roche Ltd/Genentech, Inc. via Roche Thailand Ltd. S.W. reports honoraria from F. Hoffmann-La Roche Ltd. H.W., C.D., S.L.dH., and E.R. report ownership interest (stocks, stock options, patent or other intellectual property or other ownership interest excluding diversified mutual funds) in F. Hoffmann-La Roche Ltd. F.L., M.C., S.L.dH., and E.R. report employment with F. Hoffmann-La Roche Ltd. H.W. reports employment with Roche Product Development. C.D. reports employment with Roche (China) Holding Ltd. G.S. reports employment with Hangzhou Tigermed Consulting Co., Ltd.
Figures


Similar articles
-
Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial.JAMA Oncol. 2020 Mar 1;6(3):e193692. doi: 10.1001/jamaoncol.2019.3692. Epub 2020 Mar 12. JAMA Oncol. 2020. PMID: 31647503 Free PMC article. Clinical Trial.
-
5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial.Lancet Oncol. 2016 Jun;17(6):791-800. doi: 10.1016/S1470-2045(16)00163-7. Epub 2016 May 11. Lancet Oncol. 2016. PMID: 27179402 Clinical Trial.
-
Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2018 Jan;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7. Epub 2017 Nov 23. Lancet Oncol. 2018. PMID: 29175149 Clinical Trial.
-
Efficacy and safety of HER2 inhibitors in combination with or without pertuzumab for HER2-positive breast cancer: a systematic review and meta-analysis.BMC Cancer. 2019 Oct 21;19(1):973. doi: 10.1186/s12885-019-6132-0. BMC Cancer. 2019. PMID: 31638935 Free PMC article.
-
Pertuzumab in combination with trastuzumab and docetaxel for the treatment of HER2-positive metastatic or locally recurrent unresectable breast cancer.Pharmacoeconomics. 2015 Jan;33(1):13-23. doi: 10.1007/s40273-014-0206-2. Pharmacoeconomics. 2015. PMID: 25138171 Review.
Cited by
-
HER2-targeted therapies for HER2-positive early-stage breast cancer: present and future.Front Pharmacol. 2024 Sep 16;15:1446414. doi: 10.3389/fphar.2024.1446414. eCollection 2024. Front Pharmacol. 2024. PMID: 39351085 Free PMC article. Review.
-
Modeling development of breast cancer: from tumor microenvironment to preclinical applications.Front Pharmacol. 2024 Dec 4;15:1466017. doi: 10.3389/fphar.2024.1466017. eCollection 2024. Front Pharmacol. 2024. PMID: 39697553 Free PMC article. Review.
-
Expression of c-erb-B2 oncoprotein as a neoantigen strategy to repurpose anti-neu antibody therapy in a model of melanoma.Sci Rep. 2024 Oct 19;14(1):24545. doi: 10.1038/s41598-024-76209-z. Sci Rep. 2024. PMID: 39427012 Free PMC article.
-
HER2-Positive Breast Cancer-Current Treatment Management and New Therapeutic Methods for Brain Metastasis.Biomedicines. 2025 May 9;13(5):1153. doi: 10.3390/biomedicines13051153. Biomedicines. 2025. PMID: 40426980 Free PMC article. Review.
-
Comparison of pathological complete response (pCR) between short-term (<6 cycles) and long-term (≥6 cycles) neoadjuvant trastuzumab therapy for HER2-positive breast cancer: a systematic review and meta-analysis of randomized controlled trials.Gland Surg. 2025 Jun 30;14(6):1079-1090. doi: 10.21037/gs-2025-25. Epub 2025 Jun 26. Gland Surg. 2025. PMID: 40671771 Free PMC article.
References
-
- Gianni L, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. - DOI - PubMed
-
- Untch M, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J. Clin. Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

