Effect of serum concentrations of IL-6 and TNF-α on brain structure in anorexia nervosa: a combined cross-sectional and longitudinal study
- PMID: 38461330
- PMCID: PMC11319803
- DOI: 10.1038/s41386-024-01836-z
Effect of serum concentrations of IL-6 and TNF-α on brain structure in anorexia nervosa: a combined cross-sectional and longitudinal study
Abstract
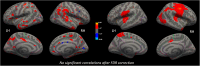
Previous studies of brain structure in anorexia nervosa (AN) have reported reduced gray matter in underweight patients, which largely normalizes upon weight gain. One underlying biological mechanism may be glial cell alterations related to low-grade inflammation. Here, we investigated relationships between brain structure as measured by magnetic resonance imaging and serum concentrations of two pro-inflammatory cytokines (interleukin-6 and tumor necrosis factor alpha) cross-sectionally in 82 underweight adolescent and young adult female patients (mean age 16.8 years; 59 of whom were observed longitudinally after short-term weight restoration; mean duration 2.8 months), 20 individuals long-term weight-recovered from AN (mean age 22.7 years) and 105 healthy control (HC) participants (mean age 17.2 years). We measured cortical thickness, subcortical volumes and local gyrification index, a measure of cortical folding. In contrast to most previous studies of cytokine concentrations in AN, we found no cross-sectional group differences (interleukin-6: p = 0.193, tumor necrosis factor alpha: p = 0.057) or longitudinal changes following weight restoration (interleukin-6: p = 0.201, tumor necrosis factor alpha: p = 0.772). As expected, widespread gray matter reductions (cortical thickness, subcortical volumes, cortical folding) were observed in underweight patients with AN compared to HC. However, we found no evidence of associations between cytokine concentrations and structural brain measures in any participant group. Furthermore, longitudinal changes in cytokine concentrations were unrelated to changes in gray matter. In conclusion, we did not identify any association between (sub-)inflammatory processes and structural brain changes in AN. Future studies are needed to elucidate which other factors besides nutritional status may contribute to brain morphological alterations.
© 2024. The Author(s).
Conflict of interest statement
VR has received payment for consulting and writing activities from Eli Lilly and Co., Novartis and Shire Pharmaceuticals/Takeda, lecture honoraria from Eli Lilly and Co., Novartis, Shire Pharmaceuticals/Takeda, and Medice Pharma, and support for research from Shire Pharmaceuticals/Takeda and Novartis. VR has carried out (and is currently carrying out) clinical trials in cooperation with Novartis, Shire Pharmaceuticals/Takeda and Otsuka. VR has no financial relationship with the organizations that sponsored the research. All other authors have nothing to disclose.
Figures



Similar articles
-
Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study.Neuroimage. 2016 Apr 15;130:214-222. doi: 10.1016/j.neuroimage.2016.02.003. Epub 2016 Feb 11. Neuroimage. 2016. PMID: 26876474
-
Dynamic Amygdala Nuclei Alterations in Relation to Weight Status in Anorexia Nervosa Are Mediated by Leptin.J Am Acad Child Adolesc Psychiatry. 2024 Jun;63(6):624-639. doi: 10.1016/j.jaac.2023.08.015. Epub 2023 Oct 3. J Am Acad Child Adolesc Psychiatry. 2024. PMID: 37797814
-
Longitudinal changes in brain-derived neurotrophic factor (BDNF) but not cytokines contribute to hippocampal recovery in anorexia nervosa above increases in body mass index.Psychol Med. 2024 Jul;54(9):2242-2253. doi: 10.1017/S0033291724000394. Epub 2024 Mar 7. Psychol Med. 2024. PMID: 38450444 Free PMC article.
-
Anorexia Nervosa in vivo cytokine production: a systematic review.Psychoneuroendocrinology. 2023 Dec;158:106390. doi: 10.1016/j.psyneuen.2023.106390. Epub 2023 Sep 15. Psychoneuroendocrinology. 2023. PMID: 37769539
-
Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations.Biol Psychiatry. 2018 Feb 1;83(3):224-234. doi: 10.1016/j.biopsych.2017.08.011. Epub 2017 Aug 24. Biol Psychiatry. 2018. PMID: 28967386 Free PMC article. Review.
Cited by
-
Cytokine and Microbiome Changes in Adolescents with Anorexia Nervosa at Admission, Discharge, and One-Year Follow-Up.Nutrients. 2024 May 23;16(11):1596. doi: 10.3390/nu16111596. Nutrients. 2024. PMID: 38892530 Free PMC article.
-
Transient patterns of advanced brain ageing in female adolescents with anorexia nervosa.Br J Psychiatry. 2024 Nov;225(5):499-505. doi: 10.1192/bjp.2024.119. Epub 2024 Dec 11. Br J Psychiatry. 2024. PMID: 39660805 Free PMC article.
-
Advances in the prevalence and treatment of depression for adolescents: a review.Front Pharmacol. 2025 May 6;16:1574574. doi: 10.3389/fphar.2025.1574574. eCollection 2025. Front Pharmacol. 2025. PMID: 40406494 Free PMC article. Review.
-
Associations between appetite loss and clinical features as well as inflammatory cytokines in adolescents with major depressive disorder.Front Psychiatry. 2025 May 14;16:1583060. doi: 10.3389/fpsyt.2025.1583060. eCollection 2025. Front Psychiatry. 2025. PMID: 40438334 Free PMC article.
References
-
- Dhopatkar N, Keeler JL, Mutwalli H, Whelan K, Treasure J, Himmerich H. Gastrointestinal symptoms, gut microbiome, probiotics and prebiotics in anorexia nervosa: a review of mechanistic rationale and clinical evidence. Psychoneuroendocrinology. 2023;147:105959. 10.1016/j.psyneuen.2022.105959 - DOI - PubMed
-
- Jésus P, Ouelaa W, François M, Riachy L, Guérin C, Aziz M, et al. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin Nutr Edinb Scotl. 2014;33:1046–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

