Amino acid profile alteration in age-related atrial fibrillation
- PMID: 38461346
- PMCID: PMC10925006
- DOI: 10.1186/s12967-024-05028-7
Amino acid profile alteration in age-related atrial fibrillation
Abstract
Background: Amino acids (AAs) are one of the primary metabolic substrates for cardiac work. The correlation between AAs and both atrial fibrillation (AF) and aging has been documented. However, the relationship between AAs and age-related AF remains unclear.
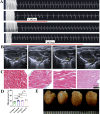
Methods: Initially, the plasma AA levels of persistent AF patients and control subjects were assessed, and the correlations between AA levels, age, and other clinical indicators were explored. Subsequently, the age-related AF mouse model was constructed and the untargeted myocardial metabolomics was conducted to detect the level of AAs and related metabolites. Additionally, the gut microbiota composition associated with age-related AF was detected by a 16S rDNA amplicon sequencing analysis on mouse fecal samples.
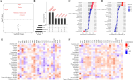
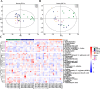
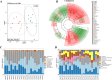
Results: Higher circulation levels of lysine (Student's t-test, P = 0.001), tyrosine (P = 0.002), glutamic acid (P = 0.008), methionine (P = 0.008), and isoleucine (P = 0.014), while a lower level of glycine (P = 0.003) were observed in persistent AF patients. The feature AAs identified by machine learning algorithms were glutamic acid and methionine. The association between AAs and age differs between AF and control subjects. Distinct patterns of AA metabolic profiles were observed in the myocardial metabolites of aged AF mice. Aged AF mice had lower levels of Betaine, L-histidine, L-alanine, L-arginine, L-Pyroglutamic acid, and L-Citrulline compared with adult AF mice. Aged AF mice also presented a different gut microbiota pattern, and its functional prediction analysis showed AA metabolism alteration.
Conclusion: This study provided a comprehensive network of AA disturbances in age-related AF from multiple dimensions, including plasma, myocardium, and gut microbiota. Disturbances of AAs may serve as AF biomarkers, and restoring their homeostasis may have potential benefits for the management of age-related AF.
Keywords: Age-related atrial fibrillation; Aging; Amino acids; Atrial fibrillation; Gut microbiota; Metabolomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no competing interests.
Figures


References
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

