Targeting vulnerable microcircuits in the ventral hippocampus of male transgenic mice to rescue Alzheimer-like social memory loss
- PMID: 38462603
- PMCID: PMC10926584
- DOI: 10.1186/s40779-024-00512-z
Targeting vulnerable microcircuits in the ventral hippocampus of male transgenic mice to rescue Alzheimer-like social memory loss
Abstract
Background: Episodic memory loss is a prominent clinical manifestation of Alzheimer's disease (AD), which is closely related to tau pathology and hippocampal impairment. Due to the heterogeneity of brain neurons, the specific roles of different brain neurons in terms of their sensitivity to tau accumulation and their contribution to AD-like social memory loss remain unclear. Therefore, further investigation is necessary.
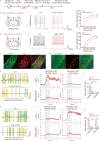
Methods: We investigated the effects of AD-like tau pathology by Tandem mass tag proteomic and phosphoproteomic analysis, social behavioural tests, hippocampal electrophysiology, immunofluorescence staining and in vivo optical fibre recording of GCaMP6f and iGABASnFR. Additionally, we utilized optogenetics and administered ursolic acid (UA) via oral gavage to examine the effects of these agents on social memory in mice.
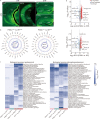
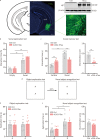
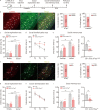
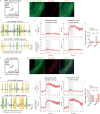
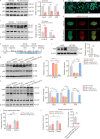
Results: The results of proteomic and phosphoproteomic analyses revealed the characteristics of ventral hippocampal CA1 (vCA1) under both physiological conditions and AD-like tau pathology. As tau progressively accumulated, vCA1, especially its excitatory and parvalbumin (PV) neurons, were fully filled with mislocated and phosphorylated tau (p-Tau). This finding was not observed for dorsal hippocampal CA1 (dCA1). The overexpression of human tau (hTau) in excitatory and PV neurons mimicked AD-like tau accumulation, significantly inhibited neuronal excitability and suppressed distinct discrimination-associated firings of these neurons within vCA1. Photoactivating excitatory and PV neurons in vCA1 at specific rhythms and time windows efficiently ameliorated tau-impaired social memory. Notably, 1 month of UA administration efficiently decreased tau accumulation via autophagy in a transcription factor EB (TFEB)-dependent manner and restored the vCA1 microcircuit to ameliorate tau-impaired social memory.
Conclusion: This study elucidated distinct protein and phosphoprotein networks between dCA1 and vCA1 and highlighted the susceptibility of the vCA1 microcircuit to AD-like tau accumulation. Notably, our novel findings regarding the efficacy of UA in reducing tau load and targeting the vCA1 microcircuit may provide a promising strategy for treating AD in the future.
Keywords: Alzheimer’s disease; Social memory; Tau protein; Transcription factor EB (TFEB); Ursolic acid; Ventral hippocampus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

