Effect of Pneumococcal Conjugate Vaccines on Viral Respiratory Infections: A Systematic Literature Review
- PMID: 38462672
- PMCID: PMC11420806
- DOI: 10.1093/infdis/jiae125
Effect of Pneumococcal Conjugate Vaccines on Viral Respiratory Infections: A Systematic Literature Review
Abstract
Background: In addition to preventing pneumococcal disease, emerging evidence indicates that pneumococcal conjugate vaccines (PCVs) might indirectly reduce viral respiratory tract infections (RTIs) by affecting pneumococcal-viral interactions.
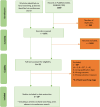
Methods: We performed a systematic review of interventional and observational studies published during 2000-2022 on vaccine efficacy/adjusted effectiveness (VE) and overall effect of PCV7, PCV9, PCV10, or PCV13 against viral RTIs.
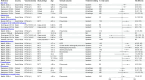
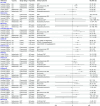
Results: Sixteen of 1671 records identified were included. Thirteen publications described effects of PCVs against viral RTIs in children. VE against influenza ranged between 41% and 86% (n = 4), except for the 2010-2011 influenza season. In a randomized controlled trial, PCV9 displayed efficacy against any viral RTI, human seasonal coronavirus, parainfluenza, and human metapneumovirus. Data in adults were limited (n = 3). PCV13 VE was 4%-25% against viral lower RTI, 32%-35% against coronavirus disease 2019 outcomes, 24%-51% against human seasonal coronavirus, and 13%-36% against influenza A lower RTI, with some 95% confidence intervals spanning zero. No protection was found against adenovirus or rhinovirus in children or adults.
Conclusions: PCVs were associated with protection against some viral RTI, with the strongest evidence for influenza in children. Limited evidence for adults was generally consistent with pediatric data. Restricting public health evaluations to confirmed pneumococcal outcomes may underestimate the full impact of PCVs.
Keywords: LRTI; lower tract respiratory infection; pneumococcal conjugate vaccine; pneumonia; viral.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. G. H., I. T. S., S. M., and M. B. are employees of P95 Epidemiology and Pharmacovigilance, which received funding from Pfizer to conduct the research described in this manuscript and for manuscript development. G. H. has also received personal fees from MSD, Sanofi Pasteur, Janssen, SNB, and Pfizer as speaker at an international meeting and as a member of advisory boards, outside the scope of the submitted work. E. M. D., L. J., C. T., and B. D. G. are employees of Pfizer, Inc, and may hold stocks or stock options. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Troeger C, Blacker B, Khalil IA, et al. . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

