T-wave morphology abnormalities in the STREAM stage 1 trial
- PMID: 38462751
- PMCID: PMC11761056
- DOI: 10.1080/14740338.2024.2322116
T-wave morphology abnormalities in the STREAM stage 1 trial
Abstract
Background: Shorter regimens for drug-resistant tuberculosis (DR-TB) have non-inferior efficacy compared with longer regimens, but QT prolongation is a concern. T-wave morphology abnormalities may be a predictor of QT prolongation.
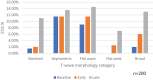
Research design and methods: STREAM Stage 1 was a randomized controlled trial in rifampicin-resistant TB, comparing short and long regimens. All participants had regular ECGs. QT/QTcF prolongation (≥500 ms or increase in ≥60 ms from baseline) was more common on the short regimen which contained high-dose moxifloxacin and clofazimine. Blinded ECGs were selected from the baseline, early (weeks 1-4), and late (weeks 12-36) time points. T-wave morphology was categorized as normal or abnormal (notched, asymmetric, flat-wave, flat peak, or broad). Differences between groups were assessed using Chi-Square tests (paired/unpaired, as appropriate).
Results: Two-hundred participants with available ECGs at relevant times were analyzed (QT prolongation group n = 82; non-prolongation group n = 118). At baseline, 23% (45/200) of participants displayed abnormal T-waves, increasing to 45% (90/200, p < 0.001) at the late time point. Abnormalities were more common in participants allocated the Short regimen (75/117, 64%) than the Long (14/38, 36.8%, p = 0.003); these occurred prior to QT/QTcF ≥500 ms in 53% of the participants (Long 2/5; Short 14/25).
Conclusions: T-wave abnormalities may help identify patients at risk of QT prolongation on DR-TB treatment.
Trial registration: The trial is registered at ClinicalTrials.gov (CT.gov identifier: NCT02409290). Current Controlled Trial number, ISRCTN78372190.
Keywords: QTcF; Rifampicin-resistant tuberculosis; T wave morphology; clofazimine; electrocardiogram; moxifloxacin.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership, or options, expert testimony, grants, or patents received or pending, or royalties.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical