Impaired polyamine metabolism causes behavioral and neuroanatomical defects in a mouse model of Snyder-Robinson syndrome
- PMID: 38463005
- PMCID: PMC11103582
- DOI: 10.1242/dmm.050639
Impaired polyamine metabolism causes behavioral and neuroanatomical defects in a mouse model of Snyder-Robinson syndrome
Abstract
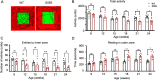
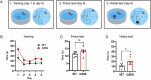
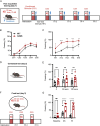
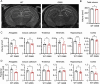
Snyder-Robinson syndrome (SRS) is a rare X-linked recessive disorder caused by a mutation in the SMS gene, which encodes spermine synthase, and aberrant polyamine metabolism. SRS is characterized by intellectual disability, thin habitus, seizure, low muscle tone/hypotonia and osteoporosis. Progress towards understanding and treating SRS requires a model that recapitulates human gene variants and disease presentations. Here, we evaluated molecular and neurological presentations in the G56S mouse model, which carries a missense mutation in the Sms gene. The lack of SMS protein in the G56S mice resulted in increased spermidine/spermine ratio, failure to thrive, short stature and reduced bone density. They showed impaired learning capacity, increased anxiety, reduced mobility and heightened fear responses, accompanied by reduced total and regional brain volumes. Furthermore, impaired mitochondrial oxidative phosphorylation was evident in G56S cerebral cortex, G56S fibroblasts and Sms-null hippocampal cells, indicating that SMS may serve as a future therapeutic target. Collectively, our study establishes the suitability of the G56S mice as a preclinical model for SRS and provides a set of molecular and functional outcome measures that can be used to evaluate therapeutic interventions for SRS.
Keywords: Mouse model; Neurological functions; Pathogenesis; Rare disease; Spermine; Spermine synthase.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Update of
-
Impaired polyamine metabolism causes behavioral and neuroanatomical defects in a novel mouse model of Snyder-Robinson Syndrome.bioRxiv [Preprint]. 2023 Feb 7:2023.01.15.524155. doi: 10.1101/2023.01.15.524155. bioRxiv. 2023. Update in: Dis Model Mech. 2024 Jun 1;17(6):dmm050639. doi: 10.1242/dmm.050639. PMID: 36711956 Free PMC article. Updated. Preprint.
References
-
- Cason, A. L., Ikeguchi, Y., Skinner, C., Wood, T. C., Holden, K. R., Lubs, H. A., Martinez, F., Simensen, R. J., Stevenson, R. E., Pegg, A. E.et al. (2003). X-linked spermine synthase gene (SMS) defect: the first polyamine deficiency syndrome. Eur. J. Hum. Genet. 11, 937-944. 10.1038/sj.ejhg.5201072 - DOI - PubMed
-
- Cervetto, C., Vergani, L., Passalacqua, M., Ragazzoni, M., Venturini, A., Cecconi, F., Berretta, N., Mercuri, N., D'Amelio, M., Maura, G.et al. (2016). Astrocyte-dependent vulnerability to excitotoxicity in spermine oxidase-overexpressing mouse. Neuromolecular Med. 18, 50-68. 10.1007/s12017-015-8377-3 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

