Accessing three-dimensional molecular diversity through benzylic C-H cross-coupling
- PMID: 38463240
- PMCID: PMC10923599
- DOI: 10.1038/s44160-023-00332-4
Accessing three-dimensional molecular diversity through benzylic C-H cross-coupling
Abstract
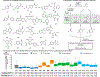
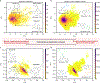
Pharmaceutical and agrochemical discovery efforts rely on robust methods for chemical synthesis that rapidly access diverse molecules1,2. Cross-coupling reactions are the most widely used synthetic methods3, but these methods typically form bonds to C(sp2)-hybridized carbon atoms (e.g., amide coupling, biaryl coupling) and lead to a prevalence of "flat" molecular structures with suboptimal physicochemical and topological properties4. Benzylic C(sp3)-H cross-coupling methods offer an appealing strategy to address this limitation by directly forming bonds to C(sp3)-hybridized carbon atoms, and emerging methods exhibit synthetic versatility that rivals conventional cross-coupling methods to access products with drug-like properties. Here, we use a virtual library of >350,000 benzylic ethers and ureas derived from benzylic C-H cross-coupling to test the widely held view that coupling at C(sp3)-hybridized carbon atoms affords products with improved three-dimensionality. The results show that the conformational rigidity of the benzylic scaffold strongly influences the product dimensionality. Products derived from flexible scaffolds often exhibit little or no improvement in three-dimensionality, unless they adopt higher energy conformations. This outcome introduces an important consideration when designing routes to topologically diverse molecular libraries. The concepts elaborated herein are validated experimentally through an informatics-guided synthesis of selected targets and the use of high-throughput experimentation to prepare a library of three-dimensional products that are broadly distributed across drug-like chemical space.
Conflict of interest statement
Competing Interests. The authors declare no competing interests.
Figures
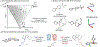



References
-
- Blakemore DC et al. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 10, 383–394 (2018). - PubMed
-
- Boström J, Brown DG, Young RJ & Keserü GM Expanding the Medicinal Chemistry Synthetic Toolbox. Nat. Rev. Drug Discov. 17, 709–727 (2018). - PubMed
-
- Brown DG & Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?: Miniperspective. J. Med. Chem. 59, 4443–4458 (2016). - PubMed
-
- Lovering F, Bikker J & Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 52, 6752–6756 (2009). - PubMed
-
- Dolle RE Historical Overview of Chemical Library Design. in Chemical Library Design (ed. Zhou JZ) 3–25 (Humana Press, 2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
