Chemometric Analysis Combined with GC × GC-FID and ESI HR-MS to Evaluate Ultralow-Sulfur Diesel Stability
- PMID: 38463272
- PMCID: PMC10918789
- DOI: 10.1021/acsomega.3c08336
Chemometric Analysis Combined with GC × GC-FID and ESI HR-MS to Evaluate Ultralow-Sulfur Diesel Stability
Abstract
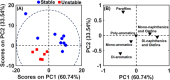
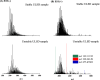
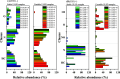
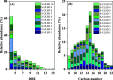
Diesel has been the most employed fuel in highway and nonhighway transportation systems. Many studies over the past years have attempted to classify diesel as a stable or unstable composition since this fuel can still degrade during storage or thermal oxidative processes. Products generated because of such degradation are the reason for the formation of soluble gums and insoluble organic particulates, which in turn cause a negative influence on engine performance. This work reports a detailed composition of nonpolar and polar compounds in many ultralow-sulfur diesel (ULSD) samples by comprehensive two-dimensional gas chromatography with a flame ionization detector (GC × GC-FID) and electrospray ionization high-resolution mass spectrometry (ESI HR-MS). In addition, chemometric approaches were applied for ULSD storage stability investigation. GC × GC-FID experiments achieved the nonpolar chemical characterization for the ULSD samples, including all main hydrocarbon classes: paraffins, mono- and dinaphthenics and olefins, and aromatics. The GC × GC-FID data combined with principal component analysis (PCA) described that the separation of the samples' concerning storage stability was mainly due to the contents of mono- and diaromatic compounds in the unstable ULSD samples. Moreover, PCA was also applied to the ESI (±) data set, and the results highlight the presence of compounds belonging to O class (natural antioxidants), which decrease the rate of oxygen consumption in the fuel, characterizing it as stable composition. The basic nitrogen compounds are mostly present in the stable ULSD samples indicating that they did not affect the stability of the fuel. On the other hand, the HC classes presented pronounced abundance among unstable ULSD samples suggesting that the fuel degradation may go through the oxidation of hydrocarbons and the formation of Ox compounds as byproducts. Furthermore, MS/MS experiments point to the formation of CcHhNnOo-like precursor species, which can react with each other and lead to the formation of gums and insoluble sediments in the fuel. In summary, the results express the potential of using the GC × GC-FID and ESI (±) Orbitrap MS techniques as valuable tools for diesel stability evaluations.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Cooper J.Fuels Europe . Refining Products for Our Everyday Life Statistical Report, 2018.
-
- US Department of Energy . Petroleum & Other Liquids.
-
- Chakravarthy R.; Acharya C.; Savalia A.; Naik G. N.; Das A. K.; Saravanan C.; Verma A.; Gudasi K. B. Property Prediction of Diesel Fuel Based on the Composition Analysis Data by Two-Dimensional Gas Chromatography. Energy Fuels 2018, 32 (3), 3760–3774. 10.1021/acs.energyfuels.7b03822. - DOI
-
- Richter F. P.; Caesar P. D.; Meisel S. L.; Offenhauer R. D. Distribution of Nitrogen in Petroleum According to Basicity. Ind. Eng. Chem. 1952, 44 (11), 2601–2605. 10.1021/ie50515a037. - DOI
-
- Mushrush G. W.; Beal E. J.; Hazlett R. N.; Hardy D. R. Chemical Basis of Instability of Shale-Derived Middle Distillate Fuels: A Model Study of the Interactive Effects between 2,5-Dimethylpyrrole and 3-Methylindole with Sulfonic and Carboxylic Acids. Energy Fuels 1991, 5 (5), 749–753. 10.1021/ef00029a022. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
