A Perspective on Electrical Stimulation and Sympathetic Regeneration in Peripheral Nerve Injuries
- PMID: 38463421
- PMCID: PMC10924057
- DOI: 10.1089/neur.2023.0133
A Perspective on Electrical Stimulation and Sympathetic Regeneration in Peripheral Nerve Injuries
Abstract
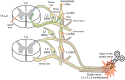
Peripheral nerve injuries (PNIs) are common and devastating. The current standard of care relies on the slow and inefficient process of nerve regeneration after surgical intervention. Electrical stimulation (ES) has been shown to both experimentally and clinically result in improved regeneration and functional recovery after PNI for motor and sensory neurons; however, its effects on sympathetic regeneration have never been studied. Sympathetic neurons are responsible for a myriad of homeostatic processes that include, but are not limited to, blood pressure, immune response, sweating, and the structural integrity of the neuromuscular junction. Almost one quarter of the axons in the sciatic nerve are from sympathetic neurons, and their importance in bodily homeostasis and the pathogenesis of neuropathic pain should not be underestimated. Therefore, as ES continues to make its way into patient care, it is not only important to understand its impact on all neuron subtypes, but also to ensure that potential adverse effects are minimized. This piece gives an overview of the effects of ES in animals models and in humans while offering a perspective on the potential effects of ES on sympathetic axon regeneration.
Keywords: axonal injury; axonal regeneration; neuroexcitation; peripheral nerve injury; regeneration.
© Tina Tian et al., 2024; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures


References
LinkOut - more resources
Full Text Sources
