This is a preprint.
Rapid hyperspectral photothermal mid-infrared spectroscopic imaging from sparse data for gynecologic cancer tissue subtyping
- PMID: 38463509
- PMCID: PMC10925386
Rapid hyperspectral photothermal mid-infrared spectroscopic imaging from sparse data for gynecologic cancer tissue subtyping
Update in
-
Rapid Hyperspectral Photothermal Mid-Infrared Spectroscopic Imaging from Sparse Data for Gynecologic Cancer Tissue Subtyping.Anal Chem. 2024 Oct 8;96(40):15880-15887. doi: 10.1021/acs.analchem.4c01093. Epub 2024 Sep 23. Anal Chem. 2024. PMID: 39312212 Free PMC article.
Abstract
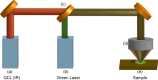
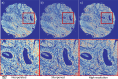
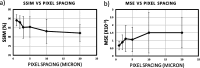
Ovarian cancer detection has traditionally relied on a multi-step process that includes biopsy, tissue staining, and morphological analysis by experienced pathologists. While widely practiced, this conventional approach suffers from several drawbacks: it is qualitative, time-intensive, and heavily dependent on the quality of staining. Mid-infrared (MIR) hyperspectral photothermal imaging is a label-free, biochemically quantitative technology that, when combined with machine learning algorithms, can eliminate the need for staining and provide quantitative results comparable to traditional histology. However, this technology is slow. This work presents a novel approach to MIR photothermal imaging that enhances its speed by an order of magnitude. Our method significantly accelerates data collection by capturing a combination of highresolution and interleaved, lower-resolution infrared band images and applying computational techniques for data interpolation. We effectively minimize data collection requirements by leveraging sparse data acquisition and employing curvelet-based reconstruction algorithms. This approach enhances imaging speed without compromising image quality and ensures robust tissue segmentation. This method resolves the longstanding trade-off between imaging resolution and data collection speed, enabling the reconstruction of high-quality, high-resolution images from undersampled datasets and achieving a 10X improvement in data acquisition time. We assessed the performance of our sparse imaging methodology using a variety of quantitative metrics, including mean squared error (MSE), structural similarity index (SSIM), and tissue subtype classification accuracies, employing both random forest and convolutional neural network (CNN) models, accompanied by Receiver Operating Characteristic (ROC) curves. Our statistically robust analysis, based on data from 100 ovarian cancer patient samples and over 65 million data points, demonstrates the method's capability to produce superior image quality and accurately distinguish between different gynecological tissue types with segmentation accuracy exceeding 95%. Our work demonstrates the feasibility of integrating rapid MIR hyperspectral photothermal imaging with machine learning in enhancing ovarian cancer tissue characterization, paving the way for quantitative, label-free, automated histopathology. It represents a significant leap forward from traditional histopathological methods, offering profound implications for cancer diagnostics and treatment decision-making.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures








Similar articles
-
Rapid Hyperspectral Photothermal Mid-Infrared Spectroscopic Imaging from Sparse Data for Gynecologic Cancer Tissue Subtyping.Anal Chem. 2024 Oct 8;96(40):15880-15887. doi: 10.1021/acs.analchem.4c01093. Epub 2024 Sep 23. Anal Chem. 2024. PMID: 39312212 Free PMC article.
-
Cervical Cancer Tissue Analysis Using Photothermal Midinfrared Spectroscopic Imaging.Chem Biomed Imaging. 2024 Jul 31;2(9):651-658. doi: 10.1021/cbmi.4c00031. eCollection 2024 Sep 23. Chem Biomed Imaging. 2024. PMID: 39328427 Free PMC article.
-
Deep convolutional neural network and IoT technology for healthcare.Digit Health. 2024 Jan 17;10:20552076231220123. doi: 10.1177/20552076231220123. eCollection 2024 Jan-Dec. Digit Health. 2024. PMID: 38250147 Free PMC article.
-
Multi-domain convolutional neural network (MD-CNN) for radial reconstruction of dynamic cardiac MRI.Magn Reson Med. 2021 Mar;85(3):1195-1208. doi: 10.1002/mrm.28485. Epub 2020 Sep 13. Magn Reson Med. 2021. PMID: 32924188
-
CT artifact correction for sparse and truncated projection data using generative adversarial networks.Med Phys. 2021 Feb;48(2):615-626. doi: 10.1002/mp.14504. Epub 2020 Dec 30. Med Phys. 2021. PMID: 32996149 Review.
References
-
- Hirschmugl C. J.; Gough K. M. Fourier transform infrared spectrochemical imaging: review of design and applications with a focal plane array and multiple beam synchrotron radiation source. Applied spectroscopy 2012, 66, 475–491. - PubMed
-
- Reddy R. K.; Bhargava R. Accurate histopathology from low signal-to-noise ratio spectroscopic imaging data. The Analyst 2010, 135, 2818–2825. - PubMed
-
- Walsh M. J.; Reddy R. K.; Bhargava R. Label-free biomedical imaging with mid-IR spectroscopy. IEEE Journal of selected topics in quantum electronics 2012, 18, 1502–1513.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
