Construction and Validation of a Novel Nomogram Predicting Recurrence in Alpha-Fetoprotein-Negative Hepatocellular Carcinoma Post-Surgery Using an Innovative Liver Function-Nutrition-Inflammation-Immune (LFNII) Score: A Bicentric Investigation
- PMID: 38463544
- PMCID: PMC10924898
- DOI: 10.2147/JHC.S451357
Construction and Validation of a Novel Nomogram Predicting Recurrence in Alpha-Fetoprotein-Negative Hepatocellular Carcinoma Post-Surgery Using an Innovative Liver Function-Nutrition-Inflammation-Immune (LFNII) Score: A Bicentric Investigation
Abstract
Purpose: We developed a nomogram based on the liver function, nutrition, inflammation, and immunity (LFNII) score to predict recurrence-free survival (RFS) post-resection in patients with hepatocellular carcinoma (HCC) exhibiting alpha-fetoprotein (AFP) negativity (AFP ≤20 ng/mL).
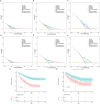
Patients and methods: Clinical data of 661 patients diagnosed with alpha-fetoprotein-negative hepatocellular carcinoma (AFP-NHCC) who underwent surgical resection at two medical centers between 2012 and 2021 were collected. A total of 462 and 199 patients served as the training and validation sets, respectively. Pre-operative blood markers were collected and analyzed for LFNII. The LFNII score was formulated using the least absolute shrinkage and selection operator Cox regression model. A nomogram model was developed using the training set to incorporate other relevant clinicopathological indicators and predict postoperative recurrence. Model discrimination was assessed using the receiver operating characteristic curve, calibration was evaluated using a calibration curve, and clinical applicability was assessed using clinical decision curve analysis. A comparison with liver cancer staging was performed using the nomogram model. Finally, a cohort study was conducted to validate our findings.
Results: We derived the LFNII scores from nine indicators. Elevated LFNII scores correlated with unfavorable clinicopathological features. The LFNII score area under the curve revealed superior predictive efficacy at 1-, 2-, and 5-year RFS intervals, with values of 0.675, 0.658, and 0.633, respectively. Multivariate Cox analysis revealed that a high LFNII score independently increased RFS risk in patients with AFP-NHCC. The C-index of the LFNII-nomogram model was 0.686 (95% confidence interval [CI], 0.651-0.721). The nomogram model's clinical application value surpassed that of standard HCC staging systems.
Conclusion: The LFNII score-derived nomogram effectively predicted the RFS of patients with AFP-NHCC after curative resection.
Keywords: alpha-fetoprotein-negative; hepatocellular carcinoma; immunity; inflammation; nutrition; recurrence-free survival.
© 2024 Zhang et al.
Conflict of interest statement
The author(s) report no conflicts of interest in this work.
Figures





References
LinkOut - more resources
Full Text Sources

