Non-alcoholic fatty liver disease: pathogenesis and models
- PMID: 38463579
- PMCID: PMC10918142
- DOI: 10.62347/KMSA5983
Non-alcoholic fatty liver disease: pathogenesis and models
Abstract
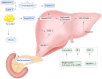
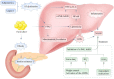
Non-alcoholic fatty liver disease (NAFLD) is a complex disease characterized by a massive accumulation of lipids in the liver, with a continuous progression of simple steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma. Non-alcoholic fatty liver disease is associated with obesity, insulin resistance, and metabolic syndrome; it is a severe public health risk and is currently the most common liver disease of the world. In addition to the fatty infiltration of the liver in non-alcoholic fatty liver disease patients, the field of liver transplantation faces similar obstacles. NAFLD and NASH primarily involve lipotoxicity, inflammation, oxidative stress, and insulin resistance. However, the precise mechanisms and treatments remain unclear. Therapeutic approaches encompass exercise, weight control, as well as treatments targeting antioxidants and anti-inflammatory pathways. The role of animal models in research has become crucial as a key tool to explore the molecular mechanisms and potential treatments for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Here, we summarized the current understanding of the pathogenesis of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis and discussed animal models commonly used in recent years.
Keywords: animal models; histopathology; non-alcoholic fatty liver disease (NAFLD); non-alcoholic steatohepatitis (NASH); pathogenesis; treatment.
AJTR Copyright © 2024.
Conflict of interest statement
None.
Figures
References
-
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9:65–90. - PMC - PubMed
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
-
- Athyros VG, Boutari C, Stavropoulos K, Anagnostis P, Imprialos KP, Doumas M, Karagiannis A. Statins: an under-appreciated asset for the prevention and the treatment of NAFLD or NASH and the related cardiovascular risk. Curr Vasc Pharmacol. 2018;16:246–253. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
