Longitudinal changes in habitual physical activity in adult people with cystic fibrosis in the presence or absence of treatment with elexacaftor/tezacaftor/ivacaftor
- PMID: 38463712
- PMCID: PMC10921570
- DOI: 10.3389/fspor.2024.1284878
Longitudinal changes in habitual physical activity in adult people with cystic fibrosis in the presence or absence of treatment with elexacaftor/tezacaftor/ivacaftor
Abstract
Background: Habitual physical activity (PA) and exercise training are accepted as important aspects of care for people with cystic fibrosis (pwCF) to improve health-related measures of physical fitness, which in turn have a positive impact on quality of life and prognosis. In the last decade, effective CFTR modulator therapies have become a promising treatment for pwCF by targeting the underlying cause of CF. This highly effective therapy improves clinical outcomes and quality of life in people with specific CFTR mutations. Little is known about the longitudinal pattern of PA or the impact of the highly effective modulator therapy with Elexacaftor/Tezacaftor/Ivacaftor (ETI) on PA in adult pwCF. This study assessed the course of device-based PA measurement in adult pwCF and evaluated the effects of ETI on habitual physical activity in those who were eligible for ETI.
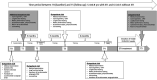
Methods: Data from adult pwCF (aged ≥18 years) were analysed at baseline and follow-up, using identical assessments at both time points. Outcome parameters were PA in steps/day and the intensity of PA. The group that received ETI was treated for an average of 33 weeks and not for the entire duration of the period. The data were collected between 2021 and 2022, following the removal of absolute pandemic restrictions/lockdowns.
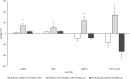
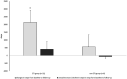
Results: Follow-up duration was 5.6 years in pwCF with ETI (ETI group, n = 21) and 6.5 years in pwCF without ETI (non-ETI group, n = 6). From baseline to follow-up, pwCF treated with ETI had a significant increase in steps/day (+25%, p = 0.019) and a non-significant increase in moderate-to-vigorous intensity time (+5.6%, p = 0.352). Conversely, individuals in the non-ETI group showed a non-significant decrease in both steps/day -3.2%, p = 0.893) and moderate-to-vigorous intensity time (-25%, p = 0.207). The ETI group showed a significant decrease in percent predicted forced expiratory volume in 1 s (ppFEV1) and FEV1 z-score before the start of ETI treatment, both of which improved significantly after therapy initiation. Body weight and body mass index also improved significantly with ETI use.
Conclusions: These data suggest that ETI treatment has a positive effect on habitual physical activity behavior in the adult pwCF studied.
Keywords: CFTR modulators; adult; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor; habitual physical activity; longitudinal effects.
© 2024 Gruber, Stehling, Blosch, Dillenhoefer, Olivier, Brinkmann, Koerner-Rettberg, Sutharsan, Mellies, Taube and Welsner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Heijerman HGM, McKone EF, Downey DG, van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. (2019) 394:1940–8. 10.1016/S0140-6736(19)32597-8 - DOI - PMC - PubMed
-
- Sutharsan S, McKone EF, Downey DG, Duckers J, MacGregor G, Tullis E, et al. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir Med. (2022) 10:267–77. 10.1016/S2213-2600(21)00454-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources

