Current perspectives on the regulatory mechanisms of sucrose accumulation in sugarcane
- PMID: 38463882
- PMCID: PMC10923725
- DOI: 10.1016/j.heliyon.2024.e27277
Current perspectives on the regulatory mechanisms of sucrose accumulation in sugarcane
Abstract
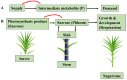
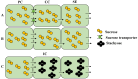
Sugars transported from leaves (source) to stems (sink) energize cell growth, elongation, and maintenance. which are regulated by a variety of genes. This review reflects progress and prospects in the regulatory mechanism for maximum sucrose accumulation, including the role of sucrose metabolizing enzymes, sugar transporters and the elucidation of post-transcriptional control of sucrose-induced regulation of translation (SIRT) in the accumulation of sucrose. The current review suggests that SIRT is emerging as a significant mechanism controlling Scbzip44 activities in response to endogenous sugar signals (via the negative feedback mechanism). Sucrose-controlled upstream open reading frame (SC-uORF) exists at the 5' leader region of Scbzip44's main ORF, which inhibits sucrose accumulation through post-transcriptional regulatory mechanisms. Sucrose transporters (SWEET1a/4a/4b/13c, TST, SUT1, SUT4 and SUT5) are crucial for sucrose translocation from source to sink. Particularly, SWEET13c was found to be a major contributor to the efflux in the transportation of stems. Tonoplast sugar transporters (TSTs), which import sucrose into the vacuole, suggest their tissue-specific role from source to sink. Sucrose cleavage has generally been linked with invertase isozymes, whereas sucrose synthase (SuSy)-catalyzed metabolism has been associated with biosynthetic processes such as UDP-Glc, cellulose, hemicellulose and other polymers. However, other two key sucrose-metabolizing enzymes, such as sucrose-6-phosphate phosphohydrolase (S6PP) and sucrose phosphate synthase (SPS) isoforms, have been linked with sucrose biosynthesis. These findings suggest that manipulation of genes, such as overexpression of SPS genes and sucrose transporter genes, silencing of the SC-uORF of Scbzip44 (removing the 5' leader region of the main ORF that is called SIRT-Insensitive) and downregulation of the invertase genes, may lead to maximum sucrose accumulation. This review provides an overview of sugarcane sucrose-regulating systems and baseline information for the development of cultivars with higher sucrose accumulation.
Keywords: Post-transcriptional factors; Source-sink communication; Sucrose accumulation; Sucrose metabolizing enzymes; Sugarcane genetic engineering.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- United States Department of Agriculture Foreign Agricultural Service. https://apps.fas.usda.gov/psdonline/circulars/sugar.pdf. (Accessed 2 August 2023).
Publication types
LinkOut - more resources
Full Text Sources

