Bioprinting of inorganic-biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery
- PMID: 38463933
- PMCID: PMC10924618
- DOI: 10.1093/nsr/nwae035
Bioprinting of inorganic-biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery
Abstract
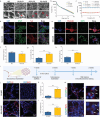
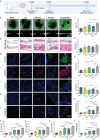
Tissue regeneration is a complicated process that relies on the coordinated effort of the nervous, vascular and immune systems. While the nervous system plays a crucial role in tissue regeneration, current tissue engineering approaches mainly focus on restoring the function of injury-related cells, neglecting the guidance provided by nerves. This has led to unsatisfactory therapeutic outcomes. Herein, we propose a new generation of engineered neural constructs from the perspective of neural induction, which offers a versatile platform for promoting multiple tissue regeneration. Specifically, neural constructs consist of inorganic biomaterials and neural stem cells (NSCs), where the inorganic biomaterials endows NSCs with enhanced biological activities including proliferation and neural differentiation. Through animal experiments, we show the effectiveness of neural constructs in repairing central nervous system injuries with function recovery. More importantly, neural constructs also stimulate osteogenesis, angiogenesis and neuromuscular junction formation, thus promoting the regeneration of bone and skeletal muscle, exhibiting its versatile therapeutic performance. These findings suggest that the inorganic-biomaterial/NSC-based neural platform represents a promising avenue for inducing the regeneration and function recovery of varying tissues and organs.
Keywords: 3D bioprinting; inorganic biomaterials; multiple tissue regeneration; neural constructs; neural stem cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures




Similar articles
-
3D bioprinted neural tissue constructs for spinal cord injury repair.Biomaterials. 2021 May;272:120771. doi: 10.1016/j.biomaterials.2021.120771. Epub 2021 Mar 25. Biomaterials. 2021. PMID: 33798962
-
Biomaterials and tissue engineering in traumatic brain injury: novel perspectives on promoting neural regeneration.Neural Regen Res. 2024 Oct 1;19(10):2157-2174. doi: 10.4103/1673-5374.391179. Epub 2023 Dec 21. Neural Regen Res. 2024. PMID: 38488550 Free PMC article.
-
3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair.Biomaterials. 2015 Dec;71:48-57. doi: 10.1016/j.biomaterials.2015.08.028. Epub 2015 Aug 17. Biomaterials. 2015. PMID: 26318816
-
Converging functionality: Strategies for 3D hybrid-construct biofabrication and the role of composite biomaterials for skeletal regeneration.Acta Biomater. 2021 Sep 15;132:188-216. doi: 10.1016/j.actbio.2021.03.008. Epub 2021 Mar 10. Acta Biomater. 2021. PMID: 33713862 Review.
-
Bioprinting stem cells: building physiological tissues one cell at a time.Am J Physiol Cell Physiol. 2020 Sep 1;319(3):C465-C480. doi: 10.1152/ajpcell.00124.2020. Epub 2020 Jul 8. Am J Physiol Cell Physiol. 2020. PMID: 32639873 Review.
Cited by
-
Bioactive Inorganic Materials for Innervated Multi-Tissue Regeneration.Adv Sci (Weinh). 2025 Apr;12(13):e2415344. doi: 10.1002/advs.202415344. Epub 2025 Feb 27. Adv Sci (Weinh). 2025. PMID: 40013907 Free PMC article. Review.
-
Biomaterials for neuroengineering: applications and challenges.Regen Biomater. 2025 Feb 21;12:rbae137. doi: 10.1093/rb/rbae137. eCollection 2025. Regen Biomater. 2025. PMID: 40007617 Free PMC article. Review.
-
Cellular and Molecular Insights into the Divergence of Neural Stem Cells on Matrigel and Poly-l-lysine Interfaces.ACS Appl Mater Interfaces. 2024 Jun 26;16(25):31922-31935. doi: 10.1021/acsami.4c02575. Epub 2024 Jun 14. ACS Appl Mater Interfaces. 2024. PMID: 38874539 Free PMC article.
-
A soft, ultra-tough and multifunctional artificial muscle for volumetric muscle loss treatment.Natl Sci Rev. 2024 Nov 22;12(2):nwae422. doi: 10.1093/nsr/nwae422. eCollection 2025 Feb. Natl Sci Rev. 2024. PMID: 39830399 Free PMC article.
References
LinkOut - more resources
Full Text Sources
