This is a preprint.
Electrophysiological dynamics of salience, default mode, and frontoparietal networks during episodic memory formation and recall: A multi-experiment iEEG replication
- PMID: 38463954
- PMCID: PMC10925291
- DOI: 10.1101/2024.02.28.582593
Electrophysiological dynamics of salience, default mode, and frontoparietal networks during episodic memory formation and recall: A multi-experiment iEEG replication
Update in
-
Electrophysiological dynamics of salience, default mode, and frontoparietal networks during episodic memory formation and recall revealed through multi-experiment iEEG replication.Elife. 2024 Nov 18;13:RP99018. doi: 10.7554/eLife.99018. Elife. 2024. PMID: 39556109 Free PMC article.
Abstract
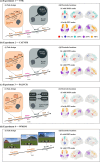
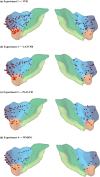
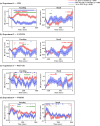
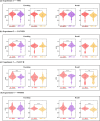
Dynamic interactions between large-scale brain networks underpin human cognitive processes, but their electrophysiological mechanisms remain elusive. The triple network model, encompassing the salience (SN), default mode (DMN), and frontoparietal (FPN) networks, provides a framework for understanding these interactions. We analyzed intracranial EEG recordings from 177 participants across four diverse episodic memory experiments, each involving encoding as well as recall phases. Phase transfer entropy analysis revealed consistently higher directed information flow from the anterior insula (AI), a key SN node, to both DMN and FPN nodes. This directed influence was significantly stronger during memory tasks compared to resting-state, highlighting the AI's task-specific role in coordinating large-scale network interactions. This pattern persisted across externally-driven memory encoding and internally-governed free recall. Control analyses using the inferior frontal gyrus (IFG) showed an inverse pattern, with DMN and FPN exerting higher influence on IFG, underscoring the AI's unique role. We observed task-specific suppression of high-gamma power in the posterior cingulate cortex/precuneus node of the DMN during memory encoding, but not recall. Crucially, these results were replicated across all four experiments spanning verbal and spatial memory domains with high Bayes replication factors. Our findings advance understanding of how coordinated neural network interactions support memory processes, highlighting the AI's critical role in orchestrating large-scale brain network dynamics during both memory encoding and retrieval. By elucidating the electrophysiological basis of triple network interactions in episodic memory, our study provides insights into neural circuit dynamics underlying memory function and offer a framework for investigating network disruptions in memory-related disorders.
Keywords: Human intracranial EEG; attentional control; default-mode network; episodic memory; frontoparietal network; human insula; salience network; triple-network model.
Conflict of interest statement
Conflict of interest statement: The authors declare no competing financial interests.
Figures

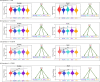
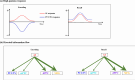
Similar articles
-
Electrophysiological dynamics of salience, default mode, and frontoparietal networks during episodic memory formation and recall revealed through multi-experiment iEEG replication.Elife. 2024 Nov 18;13:RP99018. doi: 10.7554/eLife.99018. Elife. 2024. PMID: 39556109 Free PMC article.
-
Electrophysiological foundations of the human default-mode network revealed by intracranial-EEG recordings during resting-state and cognition.Neuroimage. 2022 Apr 15;250:118927. doi: 10.1016/j.neuroimage.2022.118927. Epub 2022 Jan 21. Neuroimage. 2022. PMID: 35074503 Free PMC article.
-
Spatiotemporal Integrity and Spontaneous Nonlinear Dynamic Properties of the Salience Network Revealed by Human Intracranial Electrophysiology: A Multicohort Replication.Cereb Cortex. 2020 Sep 3;30(10):5309-5321. doi: 10.1093/cercor/bhaa111. Cereb Cortex. 2020. PMID: 32426806 Free PMC article.
-
The role of the salience network in cognitive and affective deficits.Front Hum Neurosci. 2023 Mar 20;17:1133367. doi: 10.3389/fnhum.2023.1133367. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37020493 Free PMC article. Review.
-
Convergent functional change of frontoparietal network in obsessive-compulsive disorder: a voxel-based meta-analysis.Front Psychiatry. 2024 Jul 8;15:1401623. doi: 10.3389/fpsyt.2024.1401623. eCollection 2024. Front Psychiatry. 2024. PMID: 39041046 Free PMC article. Review.
References
-
- Atkinson R. C., & Shiffrin R. M. (1968). Human memory: a proposed system and its control processes. Psychology of Learning and Motivation, 2, 89–195.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
