This is a preprint.
Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity
- PMID: 38464030
- PMCID: PMC10925330
- DOI: 10.1101/2024.03.01.582964
Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity
Update in
-
Phosphatidic acid is an endogenous negative regulator of PIEZO2 channels and mechanical sensitivity.Nat Commun. 2024 Aug 15;15(1):7020. doi: 10.1038/s41467-024-51181-4. Nat Commun. 2024. PMID: 39147733 Free PMC article.
Abstract
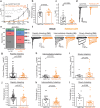
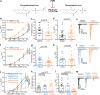
Mechanosensitive PIEZO2 ion channels play roles in touch, proprioception, and inflammatory pain. Currently, there are no small molecule inhibitors that selectively inhibit PIEZO2 over PIEZO1. The TMEM120A protein was shown to inhibit PIEZO2 while leaving PIEZO1 unaffected. Here we find that TMEM120A expression elevates cellular levels of phosphatidic acid and lysophosphatidic acid (LPA), aligning with its structural resemblance to lipid-modifying enzymes. Intracellular application of phosphatidic acid or LPA inhibited PIEZO2, but not PIEZO1 activity. Extended extracellular exposure to the non-hydrolyzable phosphatidic acid and LPA analogue carbocyclic phosphatidic acid (ccPA) also inhibited PIEZO2. Optogenetic activation of phospholipase D (PLD), a signaling enzyme that generates phosphatidic acid, inhibited PIEZO2, but not PIEZO1. Conversely, inhibiting PLD led to increased PIEZO2 activity and increased mechanical sensitivity in mice in behavioral experiments. These findings unveil lipid regulators that selectively target PIEZO2 over PIEZO1, and identify the PLD pathway as a regulator of PIEZO2 activity.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
