This is a preprint.
Structural Basis for Expanded Substrate Speci fi cities of Human Long Chain Acyl-CoA Dehydrogenase and Related Acyl- CoA Dehydrogenases
- PMID: 38464032
- PMCID: PMC10925408
- DOI: 10.21203/rs.3.rs-3980524/v1
Structural Basis for Expanded Substrate Speci fi cities of Human Long Chain Acyl-CoA Dehydrogenase and Related Acyl- CoA Dehydrogenases
Update in
-
Structural basis for expanded substrate specificities of human long chain acyl-CoA dehydrogenase and related acyl-CoA dehydrogenases.Sci Rep. 2024 Jun 5;14(1):12976. doi: 10.1038/s41598-024-63027-6. Sci Rep. 2024. PMID: 38839792 Free PMC article.
Abstract
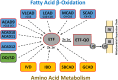
Crystal structures of human long-chain acyl-CoA dehydrogenase (LCAD) and the E291Q mutant, have been determined. These structures suggest that LCAD harbors functions beyond its historically defined role in mitochondrial β-oxidation of long and medium-chain fatty acids. LCAD is a homotetramer containing one FAD per 43kDa subunit with Glu291 as the catalytic base. The substrate binding cavity of LCAD reveals key differences which makes it specific for longer and branched chain substrates. The presence of Pro132 near the start of the E helix leads to helix unwinding that, together with adjacent smaller residues, permits binding of bulky substrates such as 3α, 7α, l2α-trihydroxy-5β-cholestan-26-oyl-CoA. This structural element is also utilized by ACAD11, a eucaryotic ACAD of unknown function, as well as bacterial ACADs known to metabolize sterol substrates. Sequence comparison suggests that ACAD10, another ACAD of unknown function, may also share this substrate specificity. These results suggest that LCAD, ACAD10, ACAD11 constitute a distinct class of eucaryotic acyl CoA dehydrogenases.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures






References
-
- Hauge J.G., Crane F.L. & Beinert H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. III. Palmityl coA dehydrogenase. The Journal of biological chemistry 219, 727–733 (1956). - PubMed
-
- Nouws J., et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell metabolism 12, 283–294 (2010). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

