This is a preprint.
Immunogenicity and safety of inactivated SARS-CoV-2 vaccine (CoronaVac) using two-dose primary protocol in children and adolescents (Immunita-002, Brazil): A phase IV six-month follow up
- PMID: 38464059
- PMCID: PMC10925469
- DOI: 10.21203/rs.3.rs-3931021/v1
Immunogenicity and safety of inactivated SARS-CoV-2 vaccine (CoronaVac) using two-dose primary protocol in children and adolescents (Immunita-002, Brazil): A phase IV six-month follow up
Update in
-
Immunogenicity and safety of CoronaVac vaccine in children and adolescents (Immunita-002, Brazil): A phase IV six-month follow up.Sci Rep. 2025 Jul 2;15(1):23040. doi: 10.1038/s41598-025-94596-9. Sci Rep. 2025. PMID: 40595400 Free PMC article. Clinical Trial.
Abstract
Introduction: Vaccines are essential for the prevention and control of several diseases, indeed, monitoring the immune response generated by vaccines is crucial. The immune response generated by vaccination against SARS-CoV-2 in children and adolescents is not well defined regarding to the intensity and medium to long-term duration of a protective immune response, which may point out the need of booster doses and might support the decisions in public health.
Objective: The study aims to evaluate the immunogenicity and safety of inactivated SARS-CoV-2 vaccine (CoronaVac) in a two-dose primary protocol in children and adolescent aging from 3 to 17 years old in Brazil.
Methods: Participants were invited to participate in the research at two public healthcare centers located in Serrana (São Paulo) and Belo Horizonte (Minas Gerais), Brazil. Participants underwent medical interviews to gather their medical history, including COVID-19 history and medical records. Physical exams were conducted, including weight, blood pressure, temperature, and pulse rate measurements. Blood samples were obtained from the participants before vaccination, 1 month after the first dose, and 1, 3, and 6 months after the second dose and were followed by a virtual platform for monitoring post-vaccination reactions and symptoms of COVID-19. SARS-CoV-2 genome from Swab samples of COVID-19 positive individuals were sequenced by NGS. Total antibodies were measured by ELISA and neutralizing antibodies to B.1 lineage and Omicron variant (BA.1) quantified by PRNT and VNT. The cellular immune response was evaluated by flow cytometry by the quantification of systemic soluble immune mediators.
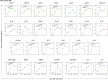
Results: The follow-up of 640 participants showed that the CoronaVac vaccine (Sinovac/Butantan Institute) was able to significantly induce the production of total IgG antibodies to SARS-CoV-2 and the production of neutralizing antibodies to B.1 lineage and Omicron variant. In addition, a robust cellular immune response was observed with wide release of pro-inflammatory and regulatory mediators in the early post-immunization moments. Adverse events recorded so far have been mild and transient except for seven serious adverse events reported on VigiMed.
Conclusions: The results indicate a robust and sustained immune response induced by the CoronaVac vaccine in children and adolescents up to six months, providing evidences to support the safety and immunogenicity of this effective immunizer.
Keywords: COVID-19; CoronaVac; SARS-CoV-2; Vaccine; antibodies kinetics; cellular markers; neutralizing antibodies.
Conflict of interest statement
MLN has received research grants from Instituto Butantan, Janssen Vaccines and Prevention B.V., Medicago R&D Inc, and Pfizer/BioNTech SE. RFQG has received grants from Instituto Butantan.Additional Declarations: No competing interests reported.
Figures








References
-
- ALVIM Renata GF et al. An affordable anti-SARS-COV-2 spike protein ELISA test for early detection of IgG seroconversion suited for large-scale surveillance studies in low-income countries. 2020.
-
- CORMAN V. et al. Diagnostic detection of Wuhan coronavirus 2019 by real-time RT-PCR-protocol and preliminary evaluation as of Jan 13, 2020. Available from www.who.int/docs/default-source/coronaviruse/wuhan-virus-assay-v1991527e..., 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

