This is a preprint.
Dietary lipid is largely deposited in skin and rapidly affects insulating properties
- PMID: 38464106
- PMCID: PMC10925457
- DOI: 10.21203/rs.3.rs-3957002/v2
Dietary lipid is largely deposited in skin and rapidly affects insulating properties
Update in
-
Dietary lipids are largely deposited in skin and rapidly affect insulating properties.Nat Commun. 2025 May 16;16(1):4570. doi: 10.1038/s41467-025-59869-x. Nat Commun. 2025. PMID: 40379673 Free PMC article.
Abstract
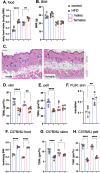
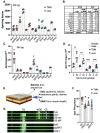
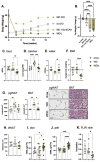
Skin has been shown to be a regulatory hub for energy expenditure and metabolism: mutations of skin lipid metabolism enzymes can change the rate of thermogenesis and susceptibility to diet-induced obesity. However, little is known about the physiological basis for this function. Here we show that the thermal properties of skin are highly reactive to diet: within three days, a high fat diet reduces heat transfer through skin. In contrast, a dietary manipulation that prevents obesity accelerates energy loss through skins. We found that skin was the largest target in a mouse body for dietary fat delivery, and that dietary triglyceride was assimilated both by epidermis and by dermal white adipose tissue. Skin from mice calorie-restricted for 3 weeks did not take up circulating lipids and showed a highly depleted stratum corneum. Dietary triglyceride acyl groups persist in skin for weeks after feeding. Using multi-modal lipid profiling, we have implicated both keratinocytes and sebocytes in the altered lipids which correlate with thermal function. In response to high fat feeding, wax diesters and ceramides accumulate, and triglycerides become more saturated. In contrast, in response to the dramatic loss of adipose tissue that accompanies restriction of the branched chain amino acid isoleucine, skin becomes more heat-permeable, resisting changes induced by Western diet feeding, with a signature of depleted signaling lipids. We propose that skin should be routinely included in physiological studies of lipid metabolism, given the size of the skin lipid reservoir and its adaptable functionality.
Conflict of interest statement
DWL has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Figures







Similar articles
-
Dietary lipids are largely deposited in skin and rapidly affect insulating properties.Nat Commun. 2025 May 16;16(1):4570. doi: 10.1038/s41467-025-59869-x. Nat Commun. 2025. PMID: 40379673 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
Publication types
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- R01 DK125513/DK/NIDDK NIH HHS/United States
- R01 DK124696/DK/NIDDK NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- R01 AG069795/AG/NIA NIH HHS/United States
- S10 OD028739/OD/NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- R01 DK125859/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R01 DK121759/DK/NIDDK NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- R01 DK133479/DK/NIDDK NIH HHS/United States
- R01 DK130879/DK/NIDDK NIH HHS/United States
- F99 AG083290/AG/NIA NIH HHS/United States
- R01 AG084156/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

