This is a preprint.
NF-κB/NLRP3 Translational Inhibition by Nanoligomer Therapy Mitigates Ethanol and Advanced Age-Related Neuroinflammation
- PMID: 38464118
- PMCID: PMC10925165
- DOI: 10.1101/2024.02.26.582114
NF-κB/NLRP3 Translational Inhibition by Nanoligomer Therapy Mitigates Ethanol and Advanced Age-Related Neuroinflammation
Update in
-
Suppression of NF-κB/NLRP3 by nanoligomer therapy mitigates ethanol and advanced age-related neuroinflammation.J Leukoc Biol. 2025 Apr 23;117(4):qiaf024. doi: 10.1093/jleuko/qiaf024. J Leukoc Biol. 2025. PMID: 40036603
Abstract
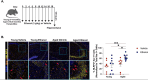
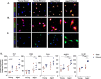
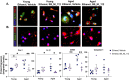
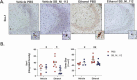
Binge alcohol use is increasing among aged adults (>65 years). Alcohol-related toxicity in aged adults is associated with neurodegeneration, yet the molecular underpinnings of age-related sensitivity to alcohol are not well described. Studies utilizing rodent models of neurodegenerative disease reveal heightened activation of Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Nod like receptor 3 (NLRP3) mediate microglia activation and associated neuronal injury. Our group, and others, have implicated hippocampal-resident microglia as key producers of inflammatory mediators, yet the link between inflammation and neurodegeneration has not been established in models of binge ethanol exposure and advanced age. Here, we report binge ethanol increased the proportion of NLRP3+ microglia in the hippocampus of aged (18-20 months) female C57BL/6N mice compared to young (3-4 months). In primary microglia, ethanol-induced expression of reactivity markers and NLRP3 inflammasome activation were more pronounced in microglia from aged mice compared to young. Making use of an NLRP3-specific inhibitor (OLT1177) and a novel brain-penetrant Nanoligomer that inhibits NF-κB and NLRP3 translation (SB_NI_112), we find ethanol-induced microglial reactivity can be attenuated by OLT1177 and SB_NI_112 in microglia from aged mice. In a model of intermittent binge ethanol exposure, SB_NI_112 prevented ethanol-mediated microglia reactivity, IL-1β production, and tau hyperphosphorylation in the hippocampus of aged mice. These data suggest early indicators of neurodegeneration occurring with advanced age and binge ethanol exposure are NF-κB- and NLRP3-dependent. Further investigation is warranted to explore the use of targeted immunosuppression via Nanoligomers to attenuate neuroinflammation after alcohol consumption in the aged.
Keywords: NLRP3 inflammasome; advanced aging; alcohol; microglia; neurodegeneration.
Conflict of interest statement
Competing Interests Authors have no competing interests to declare.
Figures



Similar articles
-
Suppression of NF-κB/NLRP3 by nanoligomer therapy mitigates ethanol and advanced age-related neuroinflammation.J Leukoc Biol. 2025 Apr 23;117(4):qiaf024. doi: 10.1093/jleuko/qiaf024. J Leukoc Biol. 2025. PMID: 40036603
-
Binge ethanol exposure in advanced age elevates neuroinflammation and early indicators of neurodegeneration and cognitive impairment in female mice.Brain Behav Immun. 2024 Feb;116:303-316. doi: 10.1016/j.bbi.2023.12.034. Epub 2023 Dec 25. Brain Behav Immun. 2024. PMID: 38151165 Free PMC article.
-
Nanoligomers targeting NF-κB and NLRP3 reduce neuroinflammation and improve cognitive function with aging and tauopathy.bioRxiv [Preprint]. 2024 Jun 4:2024.02.03.578493. doi: 10.1101/2024.02.03.578493. bioRxiv. 2024. Update in: J Neuroinflammation. 2024 Jul 27;21(1):182. doi: 10.1186/s12974-024-03182-9. PMID: 38370618 Free PMC article. Updated. Preprint.
-
Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer's disease.J Neuroimmunol. 2019 Jan 15;326:62-74. doi: 10.1016/j.jneuroim.2018.11.010. Epub 2018 Nov 20. J Neuroimmunol. 2019. PMID: 30502599 Review.
-
Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson's Disease.Front Immunol. 2021 Oct 8;12:719807. doi: 10.3389/fimmu.2021.719807. eCollection 2021. Front Immunol. 2021. PMID: 34691027 Free PMC article. Review.
References
-
- Matthews K. A., Xu W., Gaglioti A. H., Holt J. B., Croft J. B., Mack D., & McGuire L. C. . Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2019, 15 (1), 17–24, DOI: 10.1016/j.jalz.2018.06.3063. - DOI - PMC - PubMed
-
- Hou Y.; Dan X.; Babbar M.; Wei Y.; Hasselbalch S. G.; Croteau D. L.; Bohr V. A. Ageing as a risk factor for neurodegenerative disease. Nature Reviews Neurology 2019, 15 (10), 565–581. - PubMed
-
- Kanasi E.; Ayilavarapu S.; Jones J. The aging population: demographics and the biology of aging. Periodontology 2000 2016, 72 (1), 13–18. - PubMed
-
- Venkataraman A.; Kalk N.; Sewell G.; Ritchie C. W.; Lingford-Hughes A. Alcohol and Alzheimer’s disease—does alcohol dependence contribute to beta-amyloid deposition, neuroinflammation and neurodegeneration in Alzheimer’s disease? Alcohol and Alcoholism 2017, 52 (2), 151–158, DOI: 10.1093/alcalc/agw092. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
