This is a preprint.
Protective effect and molecular mechanisms of human non-neutralizing cross-reactive spike antibodies elicited by SARS-CoV-2 mRNA vaccination
- PMID: 38464151
- PMCID: PMC10925278
- DOI: 10.1101/2024.02.28.582613
Protective effect and molecular mechanisms of human non-neutralizing cross-reactive spike antibodies elicited by SARS-CoV-2 mRNA vaccination
Update in
-
Protective effect and molecular mechanisms of human non-neutralizing cross-reactive spike antibodies elicited by SARS-CoV-2 mRNA vaccination.Cell Rep. 2024 Nov 26;43(11):114922. doi: 10.1016/j.celrep.2024.114922. Epub 2024 Nov 5. Cell Rep. 2024. PMID: 39504245 Free PMC article.
Abstract
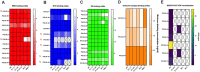
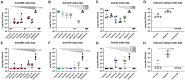
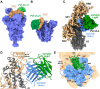
Neutralizing antibodies correlate with protection against SARS-CoV-2. Recent studies, however, show that binding antibody titers, in the absence of robust neutralizing activity, also correlate with protection from disease progression. Non-neutralizing antibodies cannot directly protect from infection but may recruit effector cells thus contribute to the clearance of infected cells. Also, they often bind conserved epitopes across multiple variants. We characterized 42 human mAbs from COVID-19 vaccinated individuals. Most of these antibodies exhibited no neutralizing activity in vitro but several non-neutralizing antibodies protected against lethal challenge with SARS-CoV-2 in different animal models. A subset of those mAbs showed a clear dependence on Fc-mediated effector functions. We determined the structures of three non-neutralizing antibodies with two targeting the RBD, and one that targeting the SD1 region. Our data confirms the real-world observation in humans that non-neutralizing antibodies to SARS-CoV-2 can be protective.
Conflict of interest statement
Conflict of interest statement The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines influenza virus vaccines and influenza virus therapeutics which list Florian Krammer as co-inventor. Dr. Simon is also listed on the SARS-CoV-2 serological assays patent. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, Castlevax, to develop SARS-CoV-2 vaccines. Florian Krammer is co-founder and scientific advisory board member of Castlevax. Florian Krammer has consulted for Merck, Curevac, Seqirus and Pfizer and is currently consulting for 3rd Rock Ventures, GSK, Gritstone and Avimex. The Krammer laboratory is also collaborating with Dynavax on influenza vaccine development. The Ellebedy laboratory has received funding under sponsored research agreements from Moderna, Emergent BioSolutions, and AbbVie. A.H.E. has received consulting and speaking fees from InBios International, Inc, Fimbrion Therapeutics, RGAX, Mubadala Investment Company, AstraZeneca, Moderna, Pfizer, GSK, Danaher, Third Rock Ventures, Goldman Sachs, and Morgan Stanley; is the founder of ImmuneBio Consulting and a recipient of royalties from licensing agreements with Abbvie and Leyden Laboratories B.V.
Figures



References
-
- The pandemic’s true death toll (2023). The Economist. https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estim....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
