This is a preprint.
Phosphate-binding pocket on cyclin B governs CDK substrate phosphorylation and mitotic timing
- PMID: 38464173
- PMCID: PMC10925351
- DOI: 10.1101/2024.02.28.582599
Phosphate-binding pocket on cyclin B governs CDK substrate phosphorylation and mitotic timing
Update in
-
Phosphate-binding pocket on cyclin B governs CDK substrate phosphorylation and mitotic timing.Nat Commun. 2025 May 8;16(1):4281. doi: 10.1038/s41467-025-59700-7. Nat Commun. 2025. PMID: 40341598 Free PMC article.
Abstract
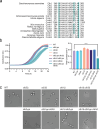
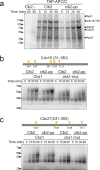
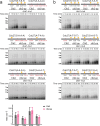
Cell cycle progression is governed by complexes of the cyclin-dependent kinases (CDKs) and their regulatory subunits cyclin and Cks1. CDKs phosphorylate hundreds of substrates, often at multiple sites. Multisite phosphorylation depends on Cks1, which binds initial priming phosphorylation sites to promote secondary phosphorylation at other sites. Here, we describe a similar role for a recently discovered phosphate-binding pocket (PP) on B-type cyclins. Mutation of the PP in Clb2, the major mitotic cyclin of budding yeast, alters bud morphology and delays the onset of anaphase. Mutation of the PP reduces multi-site phosphorylation of CDK substrates in vitro, including the Cdc16 and Cdc27 subunits of the anaphase-promoting complex/cyclosome and the Bud6 and Spa2 subunits of the polarisome. We conclude that the cyclin PP, like Cks1, controls the pattern of multisite phosphorylation on CDK substrates, thereby helping to establish the robust timing of cell-cycle events.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures

References
-
- Ubersax J. A. et al. Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 (2003). - PubMed
-
- Loog M. & Morgan D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434, 104–108 (2005). - PubMed
-
- Coudreuse D. & Nurse P. Driving the cell cycle with a minimal CDK control network. Nature 468, 1074–9 (2010). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
