This is a preprint.
Biological Effects of Corticosteroids on Pneumococcal Pneumonia in Mice and Humans
- PMID: 38464245
- PMCID: PMC10925444
- DOI: 10.21203/rs.3.rs-3962861/v1
Biological Effects of Corticosteroids on Pneumococcal Pneumonia in Mice and Humans
Update in
-
Biological effects of corticosteroids on pneumococcal pneumonia in Mice-translational significance.Crit Care. 2024 May 29;28(1):185. doi: 10.1186/s13054-024-04956-6. Crit Care. 2024. PMID: 38807178 Free PMC article.
Abstract
Background: Streptococcus pneumoniae is the most common bacterial cause of community acquired pneumonia and the acute respiratory distress syndrome (ARDS). Some clinical trials have demonstrated a beneficial effect of corticosteroid therapy in community acquired pneumonia, COVID-19, and ARDS, but the mechanisms of this benefit remain unclear. The objective of this study was to investigate the effects of corticosteroids on the pulmonary biology of pneumococcal pneumonia in an observational cohort of mechanically ventilated patients and in a mouse model of bacterial pneumonia with Streptococcus pneumoniae.
Methods: We studied gene expression with lower respiratory tract transcriptomes from a cohort of mechanically ventilated patients and in mice. We also carried out comprehensive physiologic, biochemical, and histological analyses in mice to identify the mechanisms of lung injury in Streptococcus pneumoniae with and without adjunctive steroid therapy.
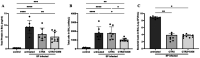
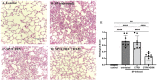
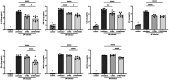
Results: Transcriptomic analysis identified pleiotropic effects of steroid therapy on the lower respiratory tract in critically ill patients with pneumococcal pneumonia, findings that were reproducible in mice. In mice with pneumonia, dexamethasone in combination with ceftriaxone reduced (1) pulmonary edema formation, (2) alveolar protein permeability, (3) proinflammatory cytokine release, (4) histopathologic lung injury score, and (5) hypoxemia but did not increase bacterial burden.
Conclusions: The gene expression studies in patients and in the mice support the clinical relevance of the mouse studies, which replicate several features of pneumococcal pneumonia and steroid therapy in humans. In combination with appropriate antibiotic therapy in mice, treatment of pneumococcal pneumonia with steroid therapy reduced hypoxemia, pulmonary edema, lung permeability, and histologic criteria of lung injury, and also altered inflammatory responses at the protein and gene expression level. The results from these studies provide evidence for the mechanisms that may explain the beneficial effects of glucocorticoid therapy in patients with community acquired pneumonia from Streptococcus Pneumoniae.
Keywords: Acute respiratory distress syndrome; Glucocorticoids; Pneumonia; Streptococcal infections.
Conflict of interest statement
-Competing interests The authors declare that they have no competing interests.
Figures


References
-
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z et al.: Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Infectious Diseases 2018, 18(11):1191–1210. - PMC - PubMed
-
- Ortqvist A, Hedlund J, Kalin M: Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med 2005, 26(6):563–574. - PubMed
-
- File TM, Ramirez JA: Community-Acquired Pneumonia. New England Journal of Medicine 2023, 389(7):632–641. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

