This is a preprint.
Noradrenergic signaling controls Alzheimer's disease pathology via activation of microglial β2 adrenergic receptors
- PMID: 38464247
- PMCID: PMC10925421
- DOI: 10.21203/rs.3.rs-3976896/v1
Noradrenergic signaling controls Alzheimer's disease pathology via activation of microglial β2 adrenergic receptors
Update in
-
Noradrenergic signaling controls Alzheimer's disease pathology via activation of microglial β2 adrenergic receptors.Brain Behav Immun. 2025 Aug;128:307-322. doi: 10.1016/j.bbi.2025.04.022. Epub 2025 Apr 15. Brain Behav Immun. 2025. PMID: 40245958
Abstract
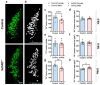
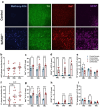
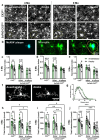
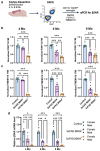
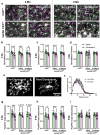
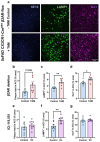
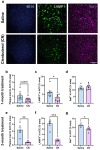
Norepinephrine (NE) is a potent anti-inflammatory agent in the brain. In Alzheimer's disease (AD), the loss of NE signaling heightens neuroinflammation and exacerbates amyloid pathology. NE inhibits surveillance activity of microglia, the brain's resident immune cells, via their β2 adrenergic receptors (β2ARs). Here, we investigate the role of microglial β2AR signaling in AD pathology in the 5xFAD mouse model of AD. We found that loss of cortical NE projections preceded the degeneration of NE-producing neurons and that microglia in 5xFAD mice, especially those microglia that were associated with plaques, significantly downregulated β2AR gene expression early in amyloid pathology. Importantly, dampening microglial β2AR signaling worsened plaque load and the associated neuritic damage, while stimulating microglial β2AR signaling attenuated amyloid pathology. Our results suggest that microglial β2AR could be explored as a potential therapeutic target to modify AD pathology.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures
Similar articles
-
Noradrenergic signaling controls Alzheimer's disease pathology via activation of microglial β2 adrenergic receptors.Brain Behav Immun. 2025 Aug;128:307-322. doi: 10.1016/j.bbi.2025.04.022. Epub 2025 Apr 15. Brain Behav Immun. 2025. PMID: 40245958
-
Noradrenergic signaling controls Alzheimer's disease pathology via activation of microglial β2 adrenergic receptors.bioRxiv [Preprint]. 2023 Dec 4:2023.12.01.569564. doi: 10.1101/2023.12.01.569564. bioRxiv. 2023. Update in: Brain Behav Immun. 2025 Aug;128:307-322. doi: 10.1016/j.bbi.2025.04.022. PMID: 38106167 Free PMC article. Updated. Preprint.
-
PLCG2 is associated with the inflammatory response and is induced by amyloid plaques in Alzheimer's disease.Genome Med. 2022 Feb 18;14(1):17. doi: 10.1186/s13073-022-01022-0. Genome Med. 2022. PMID: 35180881 Free PMC article.
-
Microglial activation as a hallmark of neuroinflammation in Alzheimer's disease.Metab Brain Dis. 2025 May 17;40(5):207. doi: 10.1007/s11011-025-01631-9. Metab Brain Dis. 2025. PMID: 40381069 Review.
-
Microglial activation states and their implications for Alzheimer's Disease.J Prev Alzheimers Dis. 2025 Jan;12(1):100013. doi: 10.1016/j.tjpad.2024.100013. Epub 2025 Jan 1. J Prev Alzheimers Dis. 2025. PMID: 39800461 Free PMC article. Review.
References
-
- Mann D. M., Yates P. O. & Hawkes J. The pathology of the human locus ceruleus. Clin Neuropathol 2, 1–7 (1983). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

