This is a preprint.
T cell and autoantibody profiling for primary immune regulatory disorders
- PMID: 38464255
- PMCID: PMC10925364
- DOI: 10.1101/2024.02.25.24303331
T cell and autoantibody profiling for primary immune regulatory disorders
Update in
-
T-cell and autoantibody profiling for primary immune regulatory disorders.J Allergy Clin Immunol. 2025 Jun 18:S0091-6749(25)00649-9. doi: 10.1016/j.jaci.2025.06.007. Online ahead of print. J Allergy Clin Immunol. 2025. PMID: 40541618
Abstract
Background: Limited clinical tools exist for characterizing primary immune regulatory disorders (PIRD), which are often diagnoses of exclusion. Increased CD4+CXCR5+PD1+ circulating T follicular helper (cTfh) cell percentages have been identified as a marker of active disease in some, but not all, autoimmune disorders.
Objective: To develop a diagnostic approach that combines measurements of cellular and serologic autoimmunity.
Methods: We recruited 71 controls and 101 pediatric patients with PIRD with autoimmunity. Flow cytometry was used to measure CD4+CXCR5+ T cells expressing the chemokine receptors CXCR3 and/or CCR6. IgG and IgA autoantibodies were quantified in 56 patients and 20 controls using a microarray featuring 1616 full-length, conformationally intact protein antigens. The 97.5th percentile in the controls serves as the upper limit of normal for percentages of cTfh cells, CD4+CXCR5+ T cells expressing CXCR3 and/or CCR6, and autoantibody intensity and number.
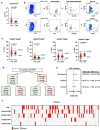
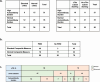
Results: We found that 27.7% of patients had increased percentages of CD4+CXCR5+PD1+ cTfh cells and 42.5% had increased percentages of CD4+CXCR5+ cells expressing CXCR3 and/or CCR6. Patients had significantly more diverse IgG and IgA autoantibodies than controls and 37.5% had increased numbers of high-titer autoantibodies. Integrating measurements of cTfh cells, CD4+CXCR5+ T cells with CXCR3 and/or CCR6, and numbers of high-titer autoantibodies had 71.4% sensitivity (95% CI: 0.5852 - 0.8158) and 85% specificity (95% CI: 0.6396 - 0.9476) for patients with PIRD compared to controls.
Conclusion: By integrating CD4+ T cell phenotyping and total burden of autoantibodies, this approach provides additional tools for the diagnosis of PIRD lacking clinical diagnostic criteria.
Keywords: T follicular helper cells; autoantibodies; autoimmunity; primary immune regulatory disorders.
Conflict of interest statement
Declaration of Interests: EMH, SC, AC, LS, MN, AB, LM, AA-M, BW, EO, SS, CY, RN, PL, OH, RCH, MD-L, AAN, ME, MG-A, CDP, BL, JC have no conflicts of interest to disclose. DHK is a consultant for Adivo Associates and Guidepoint Global. LAH received salary support from the Childhood Arthritis and Rheumatology Research Alliance, investigator-initiated research grants from Bristol Myers Squibb, and consulting fees from Sobi, Pfizer, and Adaptive Biotechnologies. TKO was an employee of BeBiopharma. JP is an employee of Sengenics. RFG receives research funding from Novartis, Sobi, and Agios and is a consultant for Agios, Sobi, and Sanofi.
Figures

References
-
- Gray PE, David C. Inborn Errors of Immunity and Autoimmune Disease. The Journal of Allergy and Clinical Immunology: In Practice. 2023. Jun;11(6):1602–22. - PubMed
-
- Sciascia S, Bizzaro N, Meroni PL, Dimitrios B, Borghi MO, Bossuyt X, et al. Autoantibodies testing in autoimmunity: Diagnostic, prognostic and classification value. Autoimmunity Reviews. 2023. Jul;22(7):103356. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
