This is a preprint.
Complement propagates visual system pathology following traumatic brain injury
- PMID: 38464312
- PMCID: PMC10925413
- DOI: 10.21203/rs.3.rs-3970621/v1
Complement propagates visual system pathology following traumatic brain injury
Update in
-
Complement propagates visual system pathology following traumatic brain injury.J Neuroinflammation. 2024 Apr 17;21(1):98. doi: 10.1186/s12974-024-03098-4. J Neuroinflammation. 2024. PMID: 38632569 Free PMC article.
Abstract
Background: Traumatic brain injury (TBI) is associated with the development of visual system disorders. Visual deficits can present with delay and worsen over time, and may be associated with an ongoing neuroinflammatory response that is known to occur after TBI. Complement activation is strongly associated with the neuroinflammatory response after TBI, but whether it contributes to vision loss after TBI is unexplored.
Methods: Acute and chronic neuroinflammatory changes within the dorsal lateral geniculate nucleus (dLGN) and retina were investigated subsequent to murine controlled unilateral cortical impact. Neuroinflammatory and histopathological data were interpreted in the context of behavioral and visual function data. To investigate the role of complement, cohorts were treated after TBI with the complement inhibitor, CR2-Crry.
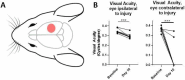
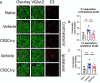
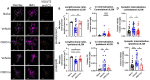
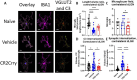
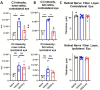
Results: At 3 days after TBI, complement C3 was deposited on retinogeniculate synapses in the dLGN both ipsilateral and contralateral to the lesion, which was reduced in CR2-Crry treated animals. This was associated with microglia morphological changes in both the ipsilateral and contralateral dLGN, with a more amoeboid phenotype in vehicle compared to CR2-Crry treated animals. Microglia in vehicle treated animals also had a greater internalized VGlut2+ synaptic volume after TBI compared to CR2-Crry treated animals. Microglia morphological changes seen acutely persisted for at least 49 days after injury. Complement inhibition also reduced microglial synaptic internalization in the contralateral dLGN and increased the association between VGLUT2 and PSD95 puncta, indicating preservation of intact synapses. Unexpectedly, there were no changes in the thickness of the inner retina, retinal nerve fiber layer or retinal ganglion layer. Pathologies were accompanied by reduced visual acuity at subacute and chronic time points after TBI, with improvement seen in CR2-Crry treated animals.
Conclusion: TBI induces complement activation within the dLGN and promotes microglial activation and synaptic internalization. Complement inhibition after TBI in a clinically relevant paradigm reduces complement activation, maintains a more surveillance-like microglia phenotype, and preserves synaptic density within the dLGN. Together, the data indicate that complement plays a key role in the development of visual deficits after TBI via complement-dependent microglial phagocytosis of synapses within the dLGN.
Conflict of interest statement
Competing interests The authors have no competing interests.
Figures



References
-
- Hac NEF, Gold DR. Neuro-Visual and Vestibular Manifestations of Concussion and Mild TBI. Curr Neurol Neurosci. 2022;22(3):219–28. - PubMed
-
- Goodrich GL, Flyg HM, Kirby JE, Chang C-Y, Martinsen GL. Mechanisms of TBI and Visual Consequences in Military and Veteran Populations. Optometry Vis Sci. 2013;90(2). - PubMed
-
- Chen B, Zhang H, Zhai Q, Li H, Wang C, Wang Y. Traumatic optic neuropathy: a review of current studies. Neurosurg Rev. 2022;45(3):1895–913. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

