Exploring monocarboxylate transporter inhibition for cancer treatment
- PMID: 38464385
- PMCID: PMC10918235
- DOI: 10.37349/etat.2024.00210
Exploring monocarboxylate transporter inhibition for cancer treatment
Abstract
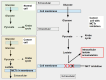
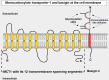
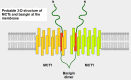
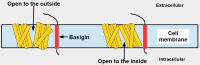
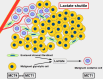
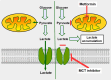
Cells are separated from the environment by a lipid bilayer membrane that is relatively impermeable to solutes. The transport of ions and small molecules across this membrane is an essential process in cell biology and metabolism. Monocarboxylate transporters (MCTs) belong to a vast family of solute carriers (SLCs) that facilitate the transport of certain hydrophylic small compounds through the bilipid cell membrane. The existence of 446 genes that code for SLCs is the best evidence of their importance. In-depth research on MCTs is quite recent and probably promoted by their role in cancer development and progression. Importantly, it has recently been realized that these transporters represent an interesting target for cancer treatment. The search for clinically useful monocarboxylate inhibitors is an even more recent field. There is limited pre-clinical and clinical experience with new inhibitors and their precise mechanism of action is still under investigation. What is common to all of them is the inhibition of lactate transport. This review discusses the structure and function of MCTs, their participation in cancer, and old and newly developed inhibitors. Some suggestions on how to improve their anticancer effects are also discussed.
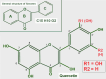
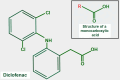
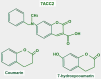
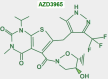
Keywords: AZD3965; Monocarboxylate transporters; diclofenac; glycolytic metabolism; lactate; lactate shuttle; quercetin.
© The Author(s) 2024.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Cellular Uptake of MCT1 Inhibitors AR-C155858 and AZD3965 and Their Effects on MCT-Mediated Transport of L-Lactate in Murine 4T1 Breast Tumor Cancer Cells.AAPS J. 2019 Jan 7;21(2):13. doi: 10.1208/s12248-018-0279-5. AAPS J. 2019. PMID: 30617815 Free PMC article.
-
Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights.Eur J Med Chem. 2020 Aug 1;199:112393. doi: 10.1016/j.ejmech.2020.112393. Epub 2020 May 1. Eur J Med Chem. 2020. PMID: 32388280 Review.
-
Role of monocarboxylate transporters in head and neck squamous cell carcinoma.Life Sci. 2021 Aug 15;279:119709. doi: 10.1016/j.lfs.2021.119709. Epub 2021 Jun 5. Life Sci. 2021. PMID: 34102188 Review.
-
In Vivo Anticancer Activity of AZD3965: A Systematic Review.Molecules. 2021 Dec 29;27(1):181. doi: 10.3390/molecules27010181. Molecules. 2021. PMID: 35011413 Free PMC article.
-
The SLC16A family of monocarboxylate transporters (MCTs)--physiology and function in cellular metabolism, pH homeostasis, and fluid transport.Curr Top Membr. 2012;70:275-311. doi: 10.1016/B978-0-12-394316-3.00009-0. Curr Top Membr. 2012. PMID: 23177990 Review.
Cited by
-
Emerging Role of Extracellular pH in Tumor Microenvironment as a Therapeutic Target for Cancer Immunotherapy.Cells. 2024 Nov 20;13(22):1924. doi: 10.3390/cells13221924. Cells. 2024. PMID: 39594672 Free PMC article. Review.
-
Targeting Lactate: An Emerging Strategy for Macrophage Regulation in Chronic Inflammation and Cancer.Biomolecules. 2024 Sep 24;14(10):1202. doi: 10.3390/biom14101202. Biomolecules. 2024. PMID: 39456135 Free PMC article. Review.
-
Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration.Int J Mol Sci. 2025 Jul 20;26(14):6966. doi: 10.3390/ijms26146966. Int J Mol Sci. 2025. PMID: 40725213 Free PMC article. Review.
References
-
- Warburg O. Über den stoffwechsel der carcinomzelle. Naturwissenschaften. 1924;12:1131–7. German. doi: 10.1007/BF01504608. - DOI
Publication types
LinkOut - more resources
Full Text Sources
