Phase 1, Dose Escalation, Nonrandomized, Open-Label, Clinical Trial Evaluating the Safety and Preliminary Efficacy of Allogenic Adipose-Derived Mesenchymal Stem Cells for Recurrent Glioblastoma: A Clinical Trial Protocol
- PMID: 38464470
- PMCID: PMC10923529
- DOI: 10.1227/neuprac.0000000000000062
Phase 1, Dose Escalation, Nonrandomized, Open-Label, Clinical Trial Evaluating the Safety and Preliminary Efficacy of Allogenic Adipose-Derived Mesenchymal Stem Cells for Recurrent Glioblastoma: A Clinical Trial Protocol
Abstract
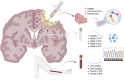
Background and objectives: Despite standard of care with maximal safe resection and chemoradiation, glioblastoma is the most common and aggressive type of primary brain cancer. Surgical resection provides a window of opportunity to locally treat gliomas while the patient is recovering, and before initiating concomitant chemoradiation. To assess the safety and establish the maximum tolerated dose of adipose-derived mesenchymal stem cells (AMSCs) for the treatment of recurrent glioblastoma (GBM). Secondary objectives are to assess the toxicity profile and long-term survival outcomes of patients enrolled in the trial. Additionally, biospecimens will be collected to explore the local and systemic responses to this therapy.
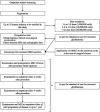
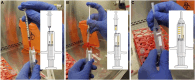
Methods: We will conduct a phase 1, dose escalated, non-randomized, open label, clinical trial of GBM patients who are undergoing surgical resection for recurrence. Up to 18 patients will receive intra-cavitary application of AMSCs encapsulated in fibrin glue during surgical resection. All patients will be followed for up to 5 years for safety and survival data. Adverse events will be recorded using the CTCAE V5.0.
Expected outcomes: This study will explore the maximum tolerated dose (MTD) of AMSCs along with the toxicity profile of this therapy in patients with recurrent GBM. Additionally, preliminary long-term survival and progression-free survival outcome analysis will be used to power further randomized studies. Lastly, CSF and blood will be obtained throughout the treatment period to investigate circulating molecular and inflammatory tumoral/stem cell markers and explore the mechanism of action of the therapeutic intervention.
Discussion: This prospective translational study will determine the initial safety and toxicity profile of local delivery of AMSCs for recurrent GBM. It will also provide additional survival metrics for future randomized trials.
Keywords: Human Mesenchymal Stem Cells; Prospective; Stem Cell; Stromal Cell; Window of Opportunity.
© The Author(s) 2023. Published by Wolters Kluwer Health, Inc. on behalf of Congress of Neurological Surgeons.
Conflict of interest statement
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Alfredo Quiñones-Hinojosa and Jordan J. Green are co-founders with equity and Managers of the startup company Dome Therapeutics.
Figures
References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114-123. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. - PubMed
-
- Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival. J Neurosurg. 2012;117(6):1032-1038. - PubMed
-
- Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German glioma network. J Clin Oncol. 2009;27(34):5743-5750. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
