Cell sheet formation enhances the therapeutic effects of adipose-derived stromal vascular fraction on urethral stricture
- PMID: 38464495
- PMCID: PMC10924207
- DOI: 10.1016/j.mtbio.2024.101012
Cell sheet formation enhances the therapeutic effects of adipose-derived stromal vascular fraction on urethral stricture
Abstract
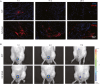
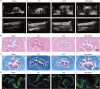
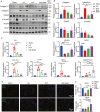
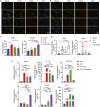
Urethral stricture (US) is a common disease in urology, lacking effective treatment options. Although injecting a stem cells suspension into the affected area has shown therapeutic benefits, challenges such as low retention rate and limited efficacy hinder the clinical application of stem cells. This study evaluates the therapeutic impact and the mechanism of adipose-derived vascular fraction (SVF) combined with cell sheet engineering technique on urethral fibrosis in a rat model of US. The results showed that SVF-cell sheets exhibit positive expression of α-SMA, CD31, CD34, Stro-1, and eNOS. In vivo study showed less collagen deposition, low urethral fibrosis, and minimal tissue alteration in the group receiving cell sheet transplantation. Furthermore, the formation of a three-dimensional (3D) tissue-like structure by the cell sheets enhances the paracrine effect of SVF, facilitates the infiltration of M2 macrophages, and suppresses the TGF-β/Smad2 pathway through HGF secretion, thereby exerting antifibrotic effects. Small animal in vivo imaging demonstrates improved retention of SVF cells at the damaged urethra site with cell sheet application. Our results suggest that SVF combined with cell sheet technology more efficiently inhibits the early stages of urethral fibrosis.
Keywords: Cell sheet; Fibrosis; Stem cell therapy; Stromal vascular fraction; Urethral stricture.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Effect of uncultured adipose-derived stromal vascular fraction on preventing urethral stricture formation in rats.Sci Rep. 2022 Mar 4;12(1):3573. doi: 10.1038/s41598-022-07472-1. Sci Rep. 2022. PMID: 35246575 Free PMC article.
-
Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy.Acta Biomater. 2017 Jun;55:131-143. doi: 10.1016/j.actbio.2017.03.034. Epub 2017 Mar 24. Acta Biomater. 2017. PMID: 28347862
-
Paracrine effect of the stromal vascular fraction containing M2 macrophages on human chondrocytes through the Smad2/3 signaling pathway.J Cell Physiol. 2022 Sep;237(9):3627-3639. doi: 10.1002/jcp.30823. Epub 2022 Jun 29. J Cell Physiol. 2022. PMID: 35766589
-
Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy.Crit Rev Eukaryot Gene Expr. 2015;25(2):145-52. doi: 10.1615/critreveukaryotgeneexpr.2015013057. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080608 Review.
-
Defining adipose tissue-derived stem cells in tissue and in culture.Histol Histopathol. 2010 Jun;25(6):807-15. doi: 10.14670/HH-25.807. Histol Histopathol. 2010. PMID: 20376787 Review.
Cited by
-
Bacterial cellulose-based scaffold with in-situ cationic micelle modification for urethral stricture disease: Sustained drug components release, cytokines recruitment, and bacterial microenvironment regulation.Bioact Mater. 2025 May 19;51:306-317. doi: 10.1016/j.bioactmat.2025.04.031. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40491686 Free PMC article.
-
A double-layer PLGA/CoI-MeHA tissue engineering scaffold for urethral reconstruction.Front Pharmacol. 2025 Feb 17;16:1555183. doi: 10.3389/fphar.2025.1555183. eCollection 2025. Front Pharmacol. 2025. PMID: 40034824 Free PMC article.
-
Local Application of Minimally Manipulated Autologous Stromal Vascular Fraction (SVF) Reduces Inflammation and Improves Bilio-Biliary Anastomosis Integrity.Int J Mol Sci. 2024 Dec 30;26(1):222. doi: 10.3390/ijms26010222. Int J Mol Sci. 2024. PMID: 39796076 Free PMC article.
-
SVF Cell Sheets as a New Multicellular Material-Based Strategy for Promoting Angiogenesis and Regeneration in Diced Cartilage Grafts.J Craniofac Surg. 2025 Apr 10;36(6):1889-98. doi: 10.1097/SCS.0000000000011358. Online ahead of print. J Craniofac Surg. 2025. PMID: 40209026 Free PMC article.
References
-
- Mundy A.R., Andrich D.E. Urethral strictures. BJU Int. 2011;107:6–26. - PubMed
-
- Rashidbenam Z., Jasman M.H., Hafez P., Tan G.H., Goh E.H., Fam X.I., Ho C.C.K., Zainuddin Z.M., Rajan R., Nor F.M., Shuhaili M.A., Kosai N.R., Imran F.H., Ng M.H. Overview of urethral reconstruction by tissue engineering: current strategies, clinical status and future direction. Tissue Eng Regen Med. 2019;16:365–384. - PMC - PubMed
-
- Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. - PMC - PubMed
LinkOut - more resources
Full Text Sources

