Novel adjuvants in allergen-specific immunotherapy: where do we stand?
- PMID: 38464539
- PMCID: PMC10920236
- DOI: 10.3389/fimmu.2024.1348305
Novel adjuvants in allergen-specific immunotherapy: where do we stand?
Abstract
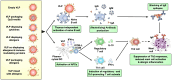
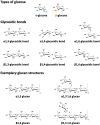
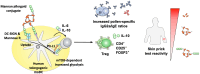
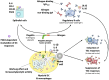
Type I hypersensitivity, or so-called type I allergy, is caused by Th2-mediated immune responses directed against otherwise harmless environmental antigens. Currently, allergen-specific immunotherapy (AIT) is the only disease-modifying treatment with the potential to re-establish clinical tolerance towards the corresponding allergen(s). However, conventional AIT has certain drawbacks, including long treatment durations, the risk of inducing allergic side effects, and the fact that allergens by themselves have a rather low immunogenicity. To improve AIT, adjuvants can be a powerful tool not only to increase the immunogenicity of co-applied allergens but also to induce the desired immune activation, such as promoting allergen-specific Th1- or regulatory responses. This review summarizes the knowledge on adjuvants currently approved for use in human AIT: aluminum hydroxide, calcium phosphate, microcrystalline tyrosine, and MPLA, as well as novel adjuvants that have been studied in recent years: oil-in-water emulsions, virus-like particles, viral components, carbohydrate-based adjuvants (QS-21, glucans, and mannan) and TLR-ligands (flagellin and CpG-ODN). The investigated adjuvants show distinct properties, such as prolonging allergen release at the injection site, inducing allergen-specific IgG production while also reducing IgE levels, as well as promoting differentiation and activation of different immune cells. In the future, better understanding of the immunological mechanisms underlying the effects of these adjuvants in clinical settings may help us to improve AIT.
Keywords: CpG; Th1/Th2 responses; adjuvant; allergen-specific immunotherapy; flagellin; mannan; type I hypersensitivity; virus-like particle.
Copyright © 2024 Lin, Zimmermann and Schülke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Allergens and Adjuvants in Allergen Immunotherapy for Immune Activation, Tolerance, and Resilience.J Allergy Clin Immunol Pract. 2021 May;9(5):1780-1789. doi: 10.1016/j.jaip.2020.12.008. Epub 2021 Mar 19. J Allergy Clin Immunol Pract. 2021. PMID: 33753052
-
State-of-the-art in marketed adjuvants and formulations in Allergen Immunotherapy: A position paper of the European Academy of Allergy and Clinical Immunology (EAACI).Allergy. 2020 Apr;75(4):746-760. doi: 10.1111/all.14134. Allergy. 2020. PMID: 31774179
-
Immunological effects of adjuvanted low-dose allergoid allergen-specific immunotherapy in experimental murine house dust mite allergy.Allergy. 2022 Mar;77(3):907-919. doi: 10.1111/all.15012. Epub 2021 Jul 26. Allergy. 2022. PMID: 34287971
-
CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance.Front Immunol. 2021 Feb 23;12:590054. doi: 10.3389/fimmu.2021.590054. eCollection 2021. Front Immunol. 2021. PMID: 33708195 Free PMC article. Review.
-
Adjuvants in Allergen-Specific Immunotherapy: Modulating and Enhancing the Immune Response.J Investig Allergol Clin Immunol. 2019;29(2):103-111. doi: 10.18176/jiaci.0349. Epub 2018 Nov 12. J Investig Allergol Clin Immunol. 2019. PMID: 30418155 Review.
Cited by
-
Allergen-Specific Immunotherapy and Trained Immunity.Allergy. 2025 Mar;80(3):677-689. doi: 10.1111/all.16423. Epub 2024 Dec 6. Allergy. 2025. PMID: 39641571 Free PMC article. Review.
-
Adverse reactions of rush subcutaneous immunotherapy using standardized house dust mite allergen extract and its prediction model construction and analysis.Asia Pac Allergy. 2025 Jun;15(2):89-98. doi: 10.5415/apallergy.0000000000000181. Epub 2025 Jun 2. Asia Pac Allergy. 2025. PMID: 40718126 Free PMC article.
-
Probiotics and other adjuvants in allergen-specific immunotherapy for food allergy: a comprehensive review.Front Allergy. 2024 Oct 10;5:1473352. doi: 10.3389/falgy.2024.1473352. eCollection 2024. Front Allergy. 2024. PMID: 39450374 Free PMC article. Review.
-
Methylated CpG ODNs from Bifidobacterium longum subsp. infantis Modulate Treg Induction and Suppress Allergic Response in a Murine Model.Int J Mol Sci. 2025 Jul 14;26(14):6755. doi: 10.3390/ijms26146755. Int J Mol Sci. 2025. PMID: 40725003 Free PMC article.
-
The clinical application of artificial intelligence in cancer precision treatment.J Transl Med. 2025 Jan 27;23(1):120. doi: 10.1186/s12967-025-06139-5. J Transl Med. 2025. PMID: 39871340 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

