Life cycle assessment for early-stage process optimization of microbial biosurfactant production using kinetic models-a case study on mannosylerythritol lipids (MEL)
- PMID: 38464544
- PMCID: PMC10920258
- DOI: 10.3389/fbioe.2024.1347452
Life cycle assessment for early-stage process optimization of microbial biosurfactant production using kinetic models-a case study on mannosylerythritol lipids (MEL)
Abstract
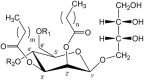
Introduction: This study assesses the environmental impacts of mannosylerythritol lipids (MELs) production for process optimization using life cycle assessment (LCA). MELs are glycolipid-type microbial biosurfactants with many possible applications based on their surface-active properties. They are generally produced by fungi from the family of Ustilaginaceae via fermentation in aerated bioreactors. The aim of our work is to accompany the development of biotechnological products at an early stage to enable environmentally sustainable process optimization. Methods: This is done by identifying hotspots and potentials for improvement based on a reliable quantification of the environmental impacts. The production processes of MELs are evaluated in a cradle-to-gate approach using the Environmental Footprint (EF) 3.1 impact assessment method. The LCA model is based on upscaled experimental data for the fermentation and purification, assuming the production at a 10 m³ scale. In the case analyzed, MELs are produced from rapeseed oil and glucose, and purified by separation, solvent extraction, and chromatography. Results: The results of the LCA show that the provision of substrates is a major source of environmental impacts and accounts for 20% of the impacts on Climate Change and more than 70% in the categories Acidification and Eutrophication. Moreover, 33% of the impacts on Climate Change is caused by the energy requirements for aeration of the bioreactor, while purification accounts for 42% of the impacts respectively. For the purification, solvents are identified as the main contributors in most impact categories. Discussion: The results illustrate the potentials for process optimization to reduce the environmental impacts of substrate requirements, enhanced bioreactor aeration, and efficient solvent use in downstream processing. By a scenario analysis, considering both experimental adaptations and prospective variations of the process, the laboratory development can be supported with further findings and hence efficiently optimized towards environmental sustainability. Moreover, the presentation of kinetic LCA results over the fermentation duration shows a novel way of calculating and visualizing results that corresponds to the way of thinking of process engineers using established environmental indicators and a detailed system analysis. Altogether, this LCA study supports and demonstrates the potential for further improvements towards more environmentally friendly produced surfactants.
Keywords: biosurfactant; biotechnology; downstream processing; fermentation; life cycle assessment (LCA); mannosylerythritol lipids (MELs); prospective LCA.
Copyright © 2024 Bippus, Briem, Beck, Zibek and Albrecht.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Aru O. O., Onwurah Ikechukwu N. E., and (2018). Life cycle assessment of the environmental impact of biosurfactant production from oil waste by a diculture of azotobacter vinelandii and Pseudomonas sp. J. Bioremediat Biodegr. 09 (03). 10.4172/2155-6199.1000435 - DOI
-
- Baccile N., Babonneau F., Banat I. M., Ciesielska K., Cuvier A.-S., Devreese B., et al. (2017). Development of a cradle-to-grave approach for acetylated acidic sophorolipid biosurfactants. ACS Sustain. Chem. Eng. 5 (1), 1186–1198. 10.1021/acssuschemeng.6b02570 - DOI
-
- Balina K., Soloha R., Suleiko A., Dubencovs K., Liepins J., Dace E. (2023). Prospective life cycle assessment of microbial sophorolipid fermentation. Fermentation 9 (9), 839. 10.3390/fermentation9090839 - DOI
-
- Beck A., Haitz F., Grunwald S., Preuss L., Rupp S., Zibek S. (2019a). Influence of microorganism and plant oils on the structure of mannosylerythritol lipid (MEL) biosurfactants revealed by a novel thin layer chromatography mass spectrometry method. J. industrial Microbiol. Biotechnol. 46 (8), 1191–1204. 10.1007/s10295-019-02194-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

