Prevalence of inappropriateness of elemene injection for hospitalized cancer patients: a multicenter retrospective study
- PMID: 38464712
- PMCID: PMC10920215
- DOI: 10.3389/fphar.2024.1334701
Prevalence of inappropriateness of elemene injection for hospitalized cancer patients: a multicenter retrospective study
Abstract
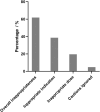
Background: Elemene injection could provide clinical benefit for the treatment of various cancers, but the clinical evidence is weak. Thus, its wide use in China has raised concerns about the appropriateness of its use. Methods: This was a multicenter retrospective study to evaluate the prevalence of inappropriateness of elemene injection for hospitalized cancer patients. Patients who met the inclusion criteria were retrospectively included, and demographic characteristics were extracted from the hospital information systems. The inappropriateness of elemene injection use was assessed using the preset criteria, and the prevalence was calculated. Multivariate logistic analysis was applied to identify any factors associated with inappropriate use. Results: A total of 275 patients were included in the analysis. The median age was 62 years, and 30.9% were females. The most common cancer was lung cancer (24.0%), and 68.2% of the patients were receiving chemotherapy. The overall prevalence of inappropriateness was 61.8%. The most common reason for inappropriateness was inappropriate indications, and the second was inappropriate doses. Age and oncological department were significant risk factors associated with inappropriate use, while lung cancer, liver cancer and admission to cardiothoracic surgery were associated with a low risk of inappropriate use. Conclusion: The prevalence of inappropriateness among hospitalized elemene injection users was high. More efforts, especially those to improve the appropriateness of indications, should be made to improve the rational use of elemene, as well as other complementary medicines. Physicians should take caution to avoid inappropriate use when prescribing drugs with limited clinical evidence.
Keywords: appropriateness; cancer; chemotherapy; elemene injection; rational.
Copyright © 2024 Cen, Jiang, Zhao, Yu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Inappropriate hospital days of a tertiary hospital in Shanghai, China.Int J Qual Health Care. 2017 Oct 1;29(5):699-704. doi: 10.1093/intqhc/mzx091. Int J Qual Health Care. 2017. PMID: 28992148
-
Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: A meta-analysis following the PRISMA guidelines.Phytomedicine. 2019 Jun;59:152787. doi: 10.1016/j.phymed.2018.12.010. Epub 2018 Dec 10. Phytomedicine. 2019. PMID: 31005810
-
Using text analysis software to identify determinants of inappropriate clinical question reporting and diagnostic procedure referrals in Reggio Emilia, Italy.BMC Health Serv Res. 2021 Jan 29;21(1):103. doi: 10.1186/s12913-021-06093-0. BMC Health Serv Res. 2021. PMID: 33514372 Free PMC article.
-
Prevalence and associated factors of inappropriate hospital admissions and days of children in a secondary hospital in Shanghai, China.PLoS One. 2022 Oct 6;17(10):e0275645. doi: 10.1371/journal.pone.0275645. eCollection 2022. PLoS One. 2022. PMID: 36201444 Free PMC article.
-
Clinical benefit and risk of elemene in cancer patients undergoing chemotherapy: a systematic review and meta-analysis.Front Pharmacol. 2023 Aug 2;14:1185987. doi: 10.3389/fphar.2023.1185987. eCollection 2023. Front Pharmacol. 2023. PMID: 37601061 Free PMC article.
References
-
- Ardoino I., Casula M., Molari G., Mucherino S., Orlando V., Menditto E., et al. (2022). Prescription appropriateness of drugs for peptic ulcer and gastro-esophageal reflux disease: baseline assessment in the LAPTOP-PPI cluster randomized trial. Front. Pharmacol. 13, 803809. 10.3389/fphar.2022.803809 - DOI - PMC - PubMed
-
- Butler A. M., Brown D. S., Durkin M. J., Sahrmann J. M., Nickel K. B., O'Neil C. A., et al. (2022). Erratum: association of inappropriate outpatient pediatric antibiotic prescriptions with adverse drug events and health care expenditures. JAMA Netw. Open 5, 2214153. 10.1001/jamanetworkopen.2022.14153 - DOI - PMC - PubMed
-
- Chen P., Li X., Zhang R., Liu S., Xiang Y., Zhang M., et al. (2020). Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 10, 5107–5119. 10.7150/thno.44705 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

