Salvia officinalis L. exerts oncostatic effects in rodent and in vitro models of breast carcinoma
- PMID: 38464730
- PMCID: PMC10921418
- DOI: 10.3389/fphar.2024.1216199
Salvia officinalis L. exerts oncostatic effects in rodent and in vitro models of breast carcinoma
Abstract
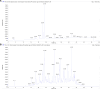
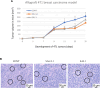
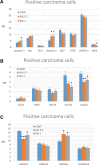
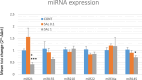
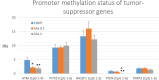
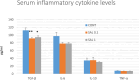
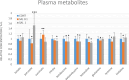
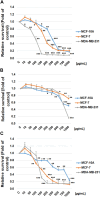
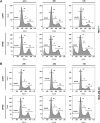
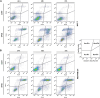
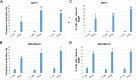
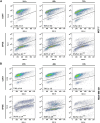
Introduction: Based on extensive data from oncology research, the use of phytochemicals or plant-based nutraceuticals is considered an innovative tool for cancer management. This research aimed to analyze the oncostatic properties of Salvia officinalis L. [Lamiaceae; Salviae officinalis herba] using animal and in vitro models of breast carcinoma (BC). Methods: The effects of dietary administered S. officinalis in two concentrations (0.1%/SAL 0.1/and 1%/SAL 1/) were assessed in both syngeneic 4T1 mouse and chemically induced rat models of BC. The histopathological and molecular evaluations of rodent carcinoma specimens were performed after the autopsy. Besides, numerous in vitro analyses using two human cancer cell lines were performed. Results and Conclusion: The dominant metabolites found in S. officinalis propylene glycol extract (SPGE) were representatives of phenolics, specifically rosmarinic, protocatechuic, and salicylic acids. Furthermore, the occurrence of triterpenoids ursolic and oleanolic acid was proved in SPGE. In a mouse model, a non-significant tumor volume decrease after S. officinalis treatment was associated with a significant reduction in the mitotic activity index of 4T1 tumors by 37.5% (SAL 0.1) and 31.5% (SAL 1) vs. controls (set as a blank group with not applied salvia in the diet). In addition, salvia at higher doses significantly decreased necrosis/whole tumor area ratio by 46% when compared to control tumor samples. In a rat chemoprevention study, S. officinalis at a higher dose significantly lengthened the latency of tumors by 8.5 days and significantly improved the high/low-grade carcinomas ratio vs. controls in both doses. Analyses of the mechanisms of anticancer activities of S. officinalis included well-validated prognostic, predictive, and diagnostic biomarkers that are applied in both oncology practice and preclinical investigation. Our assessment in vivo revealed numerous significant changes after a comparison of treated vs. untreated cancer cells. In this regard, we found an overexpression in caspase-3, an increased Bax/Bcl-2 ratio, and a decrease in MDA, ALDH1, and EpCam expression. In addition, salvia reduced TGF-β serum levels in rats (decrease in IL-6 and TNF-α levels were with borderline significance). Evaluation of epigenetic modifications in rat cancer specimens in vivo revealed a decline in the lysine methylations of H3K4m3 and an increase in lysine acetylation in H4K16ac levels in treated groups. Salvia decreased the relative levels of oncogenic miR21 and tumor-suppressive miR145 (miR210, miR22, miR34a, and miR155 were not significantly altered). The methylation of ATM and PTEN promoters was decreased after S. officinalis treatment (PITX2, RASSF1, and TIMP3 promoters were not altered). Analyzing plasma metabolomics profile in tumor-bearing rats, we found reduced levels of ketoacids derived from BCAAs after salvia treatment. In vitro analyses revealed significant anti-cancer effects of SPGE extract in MCF-7 and MDA-MB-231 cell lines (cytotoxicity, caspase-3/-7, Bcl-2, Annexin V/PI, cell cycle, BrdU, and mitochondrial membrane potential). Our study demonstrates the significant chemopreventive and treatment effects of salvia haulm using animal or in vitro BC models.
Keywords: Salvia officinalis L.; apoptosis; breast carcinoma; cancer stem cells; cell proliferation; epigenetics; human carcinoma cell lines; inflammatory cytokines.
Copyright © 2024 Kubatka, Mazurakova, Koklesova, Kuruc, Samec, Kajo, Kotorova, Adamkov, Smejkal, Svajdlenka, Dvorska, Brany, Baranovicova, Sadlonova, Mojzis and Kello.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Chemopreventive and therapeutic effects of Hippophae rhamnoides L. fruit peels evaluated in preclinical models of breast carcinoma.Front Pharmacol. 2025 Apr 30;16:1561436. doi: 10.3389/fphar.2025.1561436. eCollection 2025. Front Pharmacol. 2025. PMID: 40371330 Free PMC article.
-
Aronia melanocarpa L. fruit peels show anti-cancer effects in preclinical models of breast carcinoma: The perspectives in the chemoprevention and therapy modulation.Front Oncol. 2024 Oct 7;14:1463656. doi: 10.3389/fonc.2024.1463656. eCollection 2024. Front Oncol. 2024. PMID: 39435289 Free PMC article.
-
Rhus coriaria L. (Sumac) Demonstrates Oncostatic Activity in the Therapeutic and Preventive Model of Breast Carcinoma.Int J Mol Sci. 2020 Dec 26;22(1):183. doi: 10.3390/ijms22010183. Int J Mol Sci. 2020. PMID: 33375383 Free PMC article.
-
Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses.Molecules. 2020 Mar 19;25(6):1399. doi: 10.3390/molecules25061399. Molecules. 2020. PMID: 32204409 Free PMC article.
-
Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro.Int J Mol Sci. 2019 Apr 9;20(7):1749. doi: 10.3390/ijms20071749. Int J Mol Sci. 2019. PMID: 30970626 Free PMC article.
Cited by
-
Chemopreventive and therapeutic effects of Hippophae rhamnoides L. fruit peels evaluated in preclinical models of breast carcinoma.Front Pharmacol. 2025 Apr 30;16:1561436. doi: 10.3389/fphar.2025.1561436. eCollection 2025. Front Pharmacol. 2025. PMID: 40371330 Free PMC article.
-
The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation.Int J Mol Sci. 2024 Jul 9;25(14):7541. doi: 10.3390/ijms25147541. Int J Mol Sci. 2024. PMID: 39062784 Free PMC article.
-
Investigation of Antihypertensive Properties of Chios Mastic via Monitoring microRNA-21 Expression Levels in the Plasma of Well-Controlled Hypertensive Patients.Noncoding RNA. 2024 May 31;10(3):33. doi: 10.3390/ncrna10030033. Noncoding RNA. 2024. PMID: 38921830 Free PMC article.
-
Aronia melanocarpa L. fruit peels show anti-cancer effects in preclinical models of breast carcinoma: The perspectives in the chemoprevention and therapy modulation.Front Oncol. 2024 Oct 7;14:1463656. doi: 10.3389/fonc.2024.1463656. eCollection 2024. Front Oncol. 2024. PMID: 39435289 Free PMC article.
-
Health Benefits of Traditional Sage and Peppermint Juices: Simple Solutions for Antioxidant and Antidiabetic Support.Foods. 2025 Mar 28;14(7):1182. doi: 10.3390/foods14071182. Foods. 2025. PMID: 40238356 Free PMC article.
References
-
- Adams L. S., Phung S., Yee N., Seeram N. P., Li L., Chen S. (2010). Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 70, 3594–3605. 10.1158/0008-5472.CAN-09-3565 - DOI - PMC - PubMed
-
- Baranovicova E., Grendar M., Kalenska D., Tomascova A., Cierny D., Lehotsky J. (2018). NMR metabolomic study of blood plasma in ischemic and ischemically preconditioned rats: an increased level of ketone bodies and decreased content of glycolytic products 24 h after global cerebral ischemia. J. Physiol. Biochem. 74, 417–429. 10.1007/s13105-018-0632-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

