Unveiling the potential of Butylphthalide: inhibiting osteoclastogenesis and preventing bone loss
- PMID: 38464734
- PMCID: PMC10922197
- DOI: 10.3389/fphar.2024.1347241
Unveiling the potential of Butylphthalide: inhibiting osteoclastogenesis and preventing bone loss
Abstract
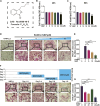
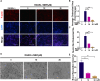
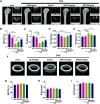
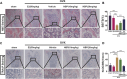
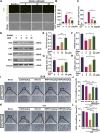
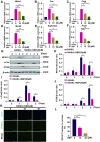
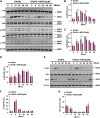
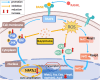
Osteoporosis, resulting from overactive osteoclasts and leading to elevated fracture risk, has emerged as a global public health concern due to the aging population. Therefore, inhibiting osteoclastogenesis and bone resorption function represents a crucial approach for preventing and treating osteoporosis. The purpose of this study was to examine the effects and molecular mechanisms of Butylphthalide (NBP) on the differentiation and function of osteoclasts induced by RANKL. Osteoclastogenesis was assessed through TRAP staining and bone slice assay. An animal model that underwent ovariectomy, simulating postmenopausal women's physiological characteristics, was established to investigate the impact of Butylphthalide on ovariectomy-induced bone loss. To delve deeper into the specific mechanisms, we employed Western blot, PCR, immunofluorescence, and immunohistochemical staining to detect the expression of proteins that are associated with the osteoclast signaling pathway. In this study, we found that Butylphthalide not only suppressed osteoclastogenesis and bone resorption in vitro but also significantly decreased TRAcP-positive osteoclasts and prevented bone loss in vivo. Further mechanistic experiments revealed that Butylphthalide reduces intracellular ROS in osteoclasts, inhibits the MAPK and NFATc1 signaling pathways, and downregulates the key genes and proteins of osteoclasts. This inhibits osteoclast formation and function. The reduction in ROS in osteoclasts is intricately linked to the activity of Butylphthalide-modulated antioxidant enzymes. Overall, NBP may offer a alternative treatment option with fewer side effects for skeletal diseases such as osteoporosis.
Keywords: Butylphthalide; ROS; osteoblast; osteoclast; osteoporosis.
Copyright © 2024 Yanbin, Yilin, Yaomin, Deshuang, Junhong, Gaofeng and Shaohui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Pseurotin A Inhibits Osteoclastogenesis and Prevents Ovariectomized-Induced Bone Loss by Suppressing Reactive Oxygen Species.Theranostics. 2019 Feb 28;9(6):1634-1650. doi: 10.7150/thno.30206. eCollection 2019. Theranostics. 2019. PMID: 31037128 Free PMC article.
-
Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways.J Ethnopharmacol. 2021 Aug 10;276:114176. doi: 10.1016/j.jep.2021.114176. Epub 2021 Apr 30. J Ethnopharmacol. 2021. PMID: 33933570
-
Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways.Bone. 2015 Jun;75:128-37. doi: 10.1016/j.bone.2015.02.017. Epub 2015 Feb 21. Bone. 2015. PMID: 25708053
-
Chaenomelis fructus inhibits osteoclast differentiation by suppressing NFATc1 expression and prevents ovariectomy-induced osteoporosis.BMC Complement Med Ther. 2020 Feb 5;20(1):35. doi: 10.1186/s12906-020-2841-9. BMC Complement Med Ther. 2020. PMID: 32024503 Free PMC article.
-
The Manganese-Bone Connection: Investigating the Role of Manganese in Bone Health.J Clin Med. 2024 Aug 9;13(16):4679. doi: 10.3390/jcm13164679. J Clin Med. 2024. PMID: 39200820 Free PMC article. Review.
References
-
- Cheon Y. H., Lee C. H., Jeong D. H., Kwak S. C., Kim S., Lee M. S., et al. (2020). Dual oxidase maturation factor 1 positively regulates RANKL-induced osteoclastogenesis via activating reactive oxygen species and TRAF6-mediated signaling. Int. J. Mol. Sci. 21 (17), 6416. 10.3390/ijms21176416 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

