Validation of the Openwater wearable optical system: cerebral hemodynamic monitoring during a breath-hold maneuver
- PMID: 38464864
- PMCID: PMC10923543
- DOI: 10.1117/1.NPh.11.1.015008
Validation of the Openwater wearable optical system: cerebral hemodynamic monitoring during a breath-hold maneuver
Abstract
Significance: Bedside cerebral blood flow (CBF) monitoring has the potential to inform and improve care for acute neurologic diseases, but technical challenges limit the use of existing techniques in clinical practice.
Aim: Here, we validate the Openwater optical system, a novel wearable headset that uses laser speckle contrast to monitor microvascular hemodynamics.
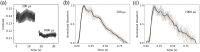
Approach: We monitored 25 healthy adults with the Openwater system and concurrent transcranial Doppler (TCD) while performing a breath-hold maneuver to increase CBF. Relative blood flow (rBF) was derived from changes in speckle contrast, and relative blood volume (rBV) was derived from changes in speckle average intensity.
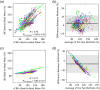
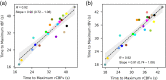
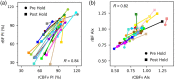
Results: A strong correlation was observed between beat-to-beat optical rBF and TCD-measured cerebral blood flow velocity (CBFv), ; the slope of the linear fit indicates good agreement, 0.87 (95% CI: 0.83 ). Beat-to-beat rBV and CBFv were also strongly correlated, , but as expected the two variables were not proportional; changes in rBV were smaller than CBFv changes, with linear fit slope of 0.18 (95% CI: 0.17 to 0.19). Further, strong agreement was found between rBF and CBFv waveform morphology and related metrics.
Conclusions: This first in vivo validation of the Openwater optical system highlights its potential as a cerebral hemodynamic monitor, but additional validation is needed in disease states.
Keywords: biomedical optics; breath-hold index; cerebral blood flow; cerebral hemodynamics; laser speckle contrast.
© 2024 The Authors.
Figures





Update of
-
Validation of the Openwater wearable optical system: cerebral hemodynamic monitoring during a breath hold maneuver.medRxiv [Preprint]. 2023 Oct 12:2023.10.11.23296612. doi: 10.1101/2023.10.11.23296612. medRxiv. 2023. Update in: Neurophotonics. 2024 Jan;11(1):015008. doi: 10.1117/1.NPh.11.1.015008. PMID: 37873126 Free PMC article. Updated. Preprint.
Similar articles
-
Validation of the Openwater wearable optical system: cerebral hemodynamic monitoring during a breath hold maneuver.medRxiv [Preprint]. 2023 Oct 12:2023.10.11.23296612. doi: 10.1101/2023.10.11.23296612. medRxiv. 2023. Update in: Neurophotonics. 2024 Jan;11(1):015008. doi: 10.1117/1.NPh.11.1.015008. PMID: 37873126 Free PMC article. Updated. Preprint.
-
Estimation of cerebral blood flow velocity during breath-hold challenge using artificial neural networks.Comput Biol Med. 2019 Dec;115:103508. doi: 10.1016/j.compbiomed.2019.103508. Epub 2019 Oct 17. Comput Biol Med. 2019. PMID: 31698237 Clinical Trial.
-
Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery.Anesth Analg. 2013 Jan;116(1):198-204. doi: 10.1213/ANE.0b013e318271fb10. Epub 2012 Dec 7. Anesth Analg. 2013. PMID: 23223100 Free PMC article.
-
Non-invasive brain stimulation in the modulation of cerebral blood flow after stroke: A systematic review of Transcranial Doppler studies.Clin Neurophysiol. 2018 Dec;129(12):2544-2551. doi: 10.1016/j.clinph.2018.09.019. Epub 2018 Oct 25. Clin Neurophysiol. 2018. PMID: 30384025
-
Identification of Pulse Onset on Cerebral Blood Flow Velocity Waveforms: A Comparative Study.Biomed Res Int. 2019 Jul 2;2019:3252178. doi: 10.1155/2019/3252178. eCollection 2019. Biomed Res Int. 2019. PMID: 31355255 Free PMC article. Review.
Cited by
-
Pre-Hospital Stroke Care beyond the MSU.Curr Neurol Neurosci Rep. 2024 Aug;24(8):315-322. doi: 10.1007/s11910-024-01351-0. Epub 2024 Jun 22. Curr Neurol Neurosci Rep. 2024. PMID: 38907812 Free PMC article. Review.
-
Compact and cost-effective laser-powered speckle contrast optical spectroscopy fiber-free device for measuring cerebral blood flow.J Biomed Opt. 2024 Jun;29(6):067001. doi: 10.1117/1.JBO.29.6.067001. Epub 2024 May 31. J Biomed Opt. 2024. PMID: 38826808 Free PMC article.
-
Portable six-channel laser speckle system for simultaneous measurement of cerebral blood flow and volume with potential applications in characterization of brain injury.Neurophotonics. 2025 Jan;12(1):015003. doi: 10.1117/1.NPh.12.1.015003. Epub 2025 Jan 24. Neurophotonics. 2025. PMID: 39867132 Free PMC article.
-
Choosing a camera and optimizing system parameters for speckle contrast optical spectroscopy.Sci Rep. 2024 May 24;14(1):11915. doi: 10.1038/s41598-024-62106-y. Sci Rep. 2024. PMID: 38789499 Free PMC article.
-
A compact and cost-effective laser-powered speckle visibility spectroscopy (SVS) device for measuring cerebral blood flow.ArXiv [Preprint]. 2024 Feb 9:arXiv:2401.16592v2. ArXiv. 2024. Update in: J Biomed Opt. 2024 Jun;29(6):067001. doi: 10.1117/1.JBO.29.6.067001. PMID: 38351942 Free PMC article. Updated. Preprint.
References
-
- Herscovitch P., Markham J., Raichle M. E., “Brain blood flow measured with intravenous . I. Theory and error analysis,” J. Nucl. Med. 24(9), 782–789 (1983).JNMEAQ - PubMed
-
- Raichle M. E., et al. , “Brain blood flow measured with intravenous . II. Implementation and validation,” J. Nucl. Med. 24(9), 790–798 (1983).JNMEAQ - PubMed
-
- Cenic A., et al. , “Dynamic CT measurement of cerebral blood flow: a validation study,” AJNR Am. J. Neuroradiol. 20(1), 63–73 (1999). - PubMed

