[Au(Np#)Cl]: Highly Reactive and Broadly Applicable Au(I)─NHC Catalysts for Alkyne π-Activation Reactions
- PMID: 38464950
- PMCID: PMC10923537
- DOI: 10.1039/d3cy00717k
[Au(Np#)Cl]: Highly Reactive and Broadly Applicable Au(I)─NHC Catalysts for Alkyne π-Activation Reactions
Abstract
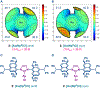
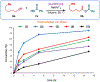
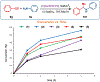
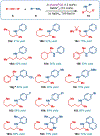
Cationic Au(I)─NHC (NHC = N-heterocyclic carbene) complexes have become an important class of catalysts for alkyne π-activation reactions in organic synthesis. In particular, these complexes are characterized by high stability of catalytic species engendered by strong σ-donation and metal backbonding. Herein, we report the synthesis and characterization of well-defined [Au(NHC)Cl] complexes featuring recently discovered IPr# family of ligands that hinge upon modular peralkylation of aniline. These ligands have been commercialized in collaboration with MilliporeSigma (IPr#: 915653; Np#: 915912; BIAN-IPr#: 916420). Evaluation of the [Au(NHC)Cl] complexes in a series of Au(I)─NHC-catalyzed π-functionalizations of alkynes, such as hydrocarboxylation, hydroamination and hydration, resulted in the identification of wingtip-flexible [Au(Np#)Cl] as a highly reactive and broadly applicable catalyst with the re-activity outperforming the classical [Au(IPr)Cl] and [Au(IPr*)Cl] complexes. The utility of this catalyst has been demonstrated in the direct late-stage derivatization of complex pharmaceuticals. Structural and computational studies were conducted to determine steric effects, frontier molecular orbitals and bond orders of this class of catalysts. Considering the attractive features of well-defined Au(I)─NHC complexes, we anticipate that this class of bulky and wingtip-flexible Au(I)─NHCs based on the modular peralkylated naphthylamine scaffold will find broad application in π-functionalization of alkynes in various areas of organic synthesis and catalysis.
Conflict of interest statement
Conflicts of interest The authors declare the following competing financial interest(s): Rutgers University has filed patent(s) on ligands and precatalysts described in this manuscript (US 63/155,492, Mar 2, 2021).
Figures







Similar articles
-
IPr# Complexes-Highly-Hindered, Sterically-Bulky Cu(I) and Ag(I) N-Heterocyclic Carbenes: Synthesis, Characterization, and Reactivity.Organometallics. 2024 Sep 21;43(19):2305-2313. doi: 10.1021/acs.organomet.4c00333. eCollection 2024 Oct 14. Organometallics. 2024. PMID: 39421292 Free PMC article.
-
[Ni(Np#)(η5-Cp)Cl]: Flexible, Sterically Bulky, Well-Defined, Highly Reactive Complex for Nickel-Catalyzed Cross-Coupling.Organometallics. 2022 Sep 26;41(18):2597-2604. doi: 10.1021/acs.organomet.2c00316. Epub 2022 Sep 2. Organometallics. 2022. PMID: 38031540 Free PMC article.
-
IPr# - highly hindered, broadly applicable N-heterocyclic carbenes.Chem Sci. 2021 Jul 2;12(31):10583-10589. doi: 10.1039/d1sc02619d. eCollection 2021 Aug 11. Chem Sci. 2021. PMID: 34447551 Free PMC article.
-
Ag-NHC Complexes in the π-Activation of Alkynes.Molecules. 2023 Jan 18;28(3):950. doi: 10.3390/molecules28030950. Molecules. 2023. PMID: 36770617 Free PMC article. Review.
-
BIAN-NHC Ligands in Transition-Metal-Catalysis: A Perfect Union of Sterically Encumbered, Electronically Tunable N-Heterocyclic Carbenes?Chemistry. 2021 Mar 8;27(14):4478-4499. doi: 10.1002/chem.202003923. Epub 2021 Jan 18. Chemistry. 2021. PMID: 32989914 Free PMC article. Review.
Cited by
-
IPr# Complexes-Highly-Hindered, Sterically-Bulky Cu(I) and Ag(I) N-Heterocyclic Carbenes: Synthesis, Characterization, and Reactivity.Organometallics. 2024 Sep 21;43(19):2305-2313. doi: 10.1021/acs.organomet.4c00333. eCollection 2024 Oct 14. Organometallics. 2024. PMID: 39421292 Free PMC article.
-
Metal-N-Heterocyclic Carbene Complexes in Buchwald-Hartwig Amination Reactions.Chem Rev. 2025 Jun 25;125(12):5349-5435. doi: 10.1021/acs.chemrev.5c00088. Epub 2025 Jun 6. Chem Rev. 2025. PMID: 40479644 Free PMC article. Review.
-
IPr*diNHC: Sterically Adaptable Dinuclear N-Heterocyclic Carbenes.Inorg Chem. 2025 Apr 28;64(16):7851-7857. doi: 10.1021/acs.inorgchem.5c01013. Epub 2025 Apr 15. Inorg Chem. 2025. PMID: 40231900 Free PMC article.
References
-
- Hopkinson MN, Richter C, Schedler M and Glorius F, Nature, 2014, 510, 485–496; - PubMed
- N-Heterocyclic Carbenes: Effective Tools for Or-ganometallic Synthesis, ed. Nolan SP, Wiley, Weinheim, 2014;
- N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, ed. Diez-Gonzalez S, RSC, Cambridge, 2016;
- Science of Synthesis: N-Heterocyclic Carbenes in Catalytic Organic Synthesis, ed. Nolan SP and Cazin CSJ, Thieme, Stuttgart, 2017;
- Huynh HV, The Organometallic Chemistry of N-Heterocyclic Carbenes, Wiley, Hoboken, 2017;
- Hopkinson MN and Glorius F, An overview of NHCs, Wiley-VCH, 2018.
-
- Lee KM, Lee CK and Lin IJB, Angew. Chem. Int. Ed, 1997, 36, 1850–1852;
- Boydston AJ, Wil-liams KA and Bielawski CW, J. Am. Chem. Soc, 2005, 127, 12496–12497; - PubMed
- Hickey JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ and Filipovska J. Am. Chem. Soc, 2008, 130, 12570–12571; - PubMed
- Hindi KM, Panzner MJ, Tessier CA, Cannon CL and Youngs WJ, Chem. Rev, 2009, 109, 3859–3884; - PMC - PubMed
- Mercs L and Al-brecht M, Chem. Soc. Rev, 2010, 39, 1903–1912; - PubMed
- Oisaki K, Li Q, Furukawa H, Czaja AU, and Yaghi OMA, J. Am. Chem.Soc, 2010, 132, 9262–9264; - PubMed
- Ranganath KVS, Kloesges J, Schafer AH and Glorius F, Angew. Chem. Int. Ed, 2010, 49, 7786–7789; - PubMed
- Lara P, Rivada-Wheelaghan O, Conejero S, Poteau R, Phillippot K and Chaudret B, Angew. Chem. Int. Ed, 2011, 50, 12080–12084; - PubMed
- Zhukhovitskiy AV, Mavros MG, Voorhis TV and Johnson JA, J. Am. Chem. Soc, 2013, 135, 7418–7421; - PubMed
- Visbal R and Gimeno MC, Chem. Soc. Rev, 2014, 43, 3551–3574. - PubMed
-
- Hermann WA, Angew. Chem. Int. Ed, 2002, 41, 1290–1309; - PubMed
- Glorius F, Top. Organomet. Chem, 2007, 21, 1–231;
- Kantchev EAB, O’Brien CJO and Organ MG, Angew. Chem. Int. Ed, 2007, 46, 2768–2813; - PubMed
- Würtz S and Glorius F, Acc. Chem. Res, 2008, 41, 1523–1533; - PubMed
- Diez-Gonzalez S, Marion N and Nolan SP, Chem. Rev, 2009, 109, 3612–3676; - PubMed
- Fortman GC and Nolan SP, Chem. Soc. Rev, 2011, 40, 5151–5169; - PubMed
- N-Heterocyclic Carbenes in Transition Metal Catalysis, ed. Cazin CSJ, Springer, New York, 2011;
- Peris E, Chem. Rev, 2018, 118, 9988–10031; - PubMed
- Sipos G and Dorta R, Coord. Chem. Rev, 2018, 375, 13–68;
- Iglesias M and Oro LA, Chem. Soc. Rev, 2018, 47, 2772–2808; - PubMed
- Zhao Q, Meng G, Nolan SP and Szostak M, Chem. Rev, 2020, 120, 1981–2048; - PMC - PubMed
- Chen C, Liu FS and Szostak M, Chem. Eur. J; 2021, 27, 4478–4499. - PMC - PubMed
-
- Nahra F, Nelson DJ and Nolan SP, Trends Chem, 2020, 2, 1096–1113.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
