Ovaries of estrogen receptor 1-deficient mice show iron overload and signs of aging
- PMID: 38464972
- PMCID: PMC10920212
- DOI: 10.3389/fendo.2024.1325386
Ovaries of estrogen receptor 1-deficient mice show iron overload and signs of aging
Abstract
Introduction: Estrogens are crucial regulators of ovarian function, mediating their signaling through binding to estrogen receptors. The disruption of the estrogen receptor 1 (Esr1) provokes infertility associated with a hemorrhagic, cystic phenotype similar to that seen in diseased or aged ovaries. Our previous study indicated the possibility of altered iron metabolism in Esr1-deficient ovaries showing massive expression of lipocalin 2, a regulator of iron homeostasis.
Methods: Therefore, we examined the consequences of depleting Esr1 in mouse ovaries, focusing on iron metabolism. For that reason, we compared ovaries of adult Esr1-deficient animals and age-matched wild type littermates.
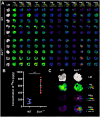
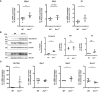
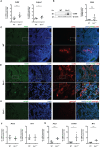
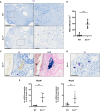
Results and discussion: We found increased iron accumulation in Esr1-deficient animals by using laser ablation inductively coupled plasma mass spectrometry. Western blot analysis and RT-qPCR confirmed that iron overload alters iron transport, storage and regulation. In addition, trivalent iron deposits in form of hemosiderin were detected in Esr1-deficient ovarian stroma. The depletion of Esr1 was further associated with an aberrant immune cell landscape characterized by the appearance of macrophage-derived multinucleated giant cells (MNGCs) and increased quantities of macrophages, particularly M2-like macrophages. Similar to reproductively aged animals, MNGCs in Esr1-deficient ovaries were characterized by iron accumulation and strong autofluorescence. Finally, deletion of Esr1 led to a significant increase in ovarian mast cells, involved in iron-mediated foam cell formation. Given that these findings are characteristics of ovarian aging, our data suggest that Esr1 deficiency triggers mechanisms similar to those associated with aging.
Keywords: ERα; Esr1; aging; estrogen receptor alpha; iron; macrophage; multinucleated giant cells; ovary.
Copyright © 2024 Schröder, Krizanac, Kim, Kessel and Weiskirchen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Expression Analysis of Lipocalin 2 (LCN2) in Reproductive and Non-Reproductive Tissues of Esr1-Deficient Mice.Int J Mol Sci. 2023 May 25;24(11):9280. doi: 10.3390/ijms24119280. Int J Mol Sci. 2023. PMID: 37298232 Free PMC article.
-
Dual roles for Id4 in the regulation of estrogen signaling in the mammary gland and ovary.Development. 2014 Aug;141(16):3159-64. doi: 10.1242/dev.108498. Epub 2014 Jul 18. Development. 2014. PMID: 25038044
-
Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely.Endocrinology. 2009 Aug;150(8):3855-62. doi: 10.1210/en.2008-1774. Epub 2009 May 7. Endocrinology. 2009. PMID: 19423761 Free PMC article.
-
Rare causes of hereditary iron overload.Semin Hematol. 2002 Oct;39(4):249-62. doi: 10.1053/shem.2002.35638. Semin Hematol. 2002. PMID: 12382200 Review.
-
[Changes of the localization and function of estrogen receptor alpha in the ovaries of postmenopausal women comparing to those of reproductive-age].Pol Merkur Lekarski. 2007 Sep;23(135):231-4. Pol Merkur Lekarski. 2007. PMID: 18080702 Review. Polish.
Cited by
-
Overview of the expression patterns and roles of Lipocalin 2 in the reproductive system.Front Endocrinol (Lausanne). 2024 Apr 5;15:1365602. doi: 10.3389/fendo.2024.1365602. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38645429 Free PMC article. Review.
-
Effect of estradiol valerate on humoral immune function in BALB/c mice after total hysterectomy.Braz J Med Biol Res. 2025 Jun 20;58:e14651. doi: 10.1590/1414-431X2025e14651. eCollection 2025. Braz J Med Biol Res. 2025. PMID: 40561276 Free PMC article.
-
Oxidative Stress, Parity History, and Remnant Follicles in the Aged Ovary: Insights on Ovarian Cancer Risk and Protection.Antioxidants (Basel). 2025 Jun 20;14(7):759. doi: 10.3390/antiox14070759. Antioxidants (Basel). 2025. PMID: 40722863 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

