Intermolecular Metal-Catalyzed C‒C Coupling of Unactivated Alcohols or Aldehydes for Convergent Ketone Construction beyond Premetalated Reagents
- PMID: 38464997
- PMCID: PMC10923551
- DOI: 10.1021/acscatal.3c02209
Intermolecular Metal-Catalyzed C‒C Coupling of Unactivated Alcohols or Aldehydes for Convergent Ketone Construction beyond Premetalated Reagents
Abstract
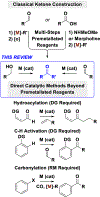
Intermolecular metal-catalyzed C‒C couplings of unactivated primary alcohols or aldehydes to form ketones are catalogued. Reactions are classified on the basis of pronucleophile. Protocols involving premetalated reagents or reactants that incorporate directing groups are not covered. These methods represent an emerging alternative to classical multi-step protocols for ketone construction that exploit premetalated reagents, and/or steps devoted to redox manipulations and carboxylic acid derivatization.
Keywords: Alcohol; Aldehyde; C-C Coupling; Green Chemistry; Ketone.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol-Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier.Acc Chem Res. 2017 Sep 19;50(9):2371-2380. doi: 10.1021/acs.accounts.7b00308. Epub 2017 Aug 9. Acc Chem Res. 2017. PMID: 28792731 Free PMC article.
-
Catalytic Enantioselective C-C Coupling of Alcohols for Polyketide Total Synthesis beyond Chiral Auxiliaries and Premetalated Reagents.Chem Rev. 2024 Dec 25;124(24):13715-13735. doi: 10.1021/acs.chemrev.4c00858. Epub 2024 Dec 6. Chem Rev. 2024. PMID: 39642170 Review.
-
Transition-Metal-Catalyzed C-C Bond Formation from C-C Activation.Acc Chem Res. 2023 Nov 7;56(21):2867-2886. doi: 10.1021/acs.accounts.3c00230. Epub 2023 Oct 26. Acc Chem Res. 2023. PMID: 37882453
-
Reductive Cross-Coupling of Unreactive Electrophiles.Acc Chem Res. 2022 Sep 6;55(17):2491-2509. doi: 10.1021/acs.accounts.2c00381. Epub 2022 Aug 11. Acc Chem Res. 2022. PMID: 35951536
-
Asymmetric Iridium-Catalyzed C-C Coupling of Chiral Diols via Site-Selective Redox-Triggered Carbonyl Addition.Top Curr Chem. 2016;372:85-101. doi: 10.1007/128_2015_651. Top Curr Chem. 2016. PMID: 26187028 Free PMC article. Review.
Cited by
-
Direct ketone synthesis from primary alcohols and alkenes enabled by a dual photo/cobalt catalysis.Nat Commun. 2024 Aug 9;15(1):6816. doi: 10.1038/s41467-024-51190-3. Nat Commun. 2024. PMID: 39122715 Free PMC article.
-
Dehomologative C-C Borylation of Aldehydes and Alcohols via a Rh-Catalyzed Dehydroformylation-Borylation Relay.J Am Chem Soc. 2025 May 21;147(20):16735-16741. doi: 10.1021/jacs.5c02181. Epub 2025 May 12. J Am Chem Soc. 2025. PMID: 40354369 Free PMC article.
-
Formate-Mediated C-C Coupling of Aryl/Vinyl Halides and Triflates: Carbonyl Arylation and Reductive Cross-Coupling.ACS Catal. 2025 May 16;15(10):8274-8283. doi: 10.1021/acscatal.5c01994. Epub 2025 May 2. ACS Catal. 2025. PMID: 40843079 Free PMC article.
References
-
- Ertl PA; Schuhmann T. Systematic Cheminformatics Analysis of Functional Groups Occurring in Natural Products. J. Nat. Prod 2019, 82, 1258–1263. - PubMed
-
- Falbe J; Bahrmann H; Lipps W; Mayer D. Alcohols, Aliphatic. In Ullman’s Encyclopedia of Industrial Chemistry, Vol. 2, Wiley-VCH: Weinheim, Germany, 2012; pp 235–261.
-
- For selected reviews on hydroformylation, see: (a)Beller M; Cornils B; Frohning CD; Kohlpaintner CW Progress in Hydroformylation and Carbonylation. J. Mol. Cat. A 1995, 104, 17–85.
- Leeuwen PWNM; Claver C. Asymmetric Hydroformylation. In Rhodium Catalyzed Hydroformylation. Catalysis by Metal Complexes; Kluwer Academic Publishers, Dordrecht, Netherlands, 2000, Vol 22, pp 107–144.
- Breit B; Seiche W. Recent Advances on Chemo-, Regio- and Stereoselective Hydroformylation. Synthesis 2001, 1, 1–36.
- Frohning CD; Kohlpaintner CW; Bohnen H-W Carbon Monoxide and Synthesis Gas Chemistry. In Applied Homogenous Catalysis with Organometallic Compounds, 2nd ed.; Wiley-VCH Weinheim, 2002, pp 29–194.
- Weissermel K; Arpe H-J Syntheses Involving Carbon Monoxide. In Industrial Organic Chemistry 4th ed.; Wiley-VCH; Weinheim, 2003, pp 127–144.
- Leeuwen PWNM, Rhodium Catalysed Hydroformylation. In Homogenous Catalysis: Understanding the Art; Kluwer Academic Publishers: Dordrecht, Netherlands, 2004, pp 139–174.
- Klosin J; Landis CR Ligands for Practical Rhodium-Catalyzed Asymmetric Hydroformylation. Acc. Chem. Res 2007, 40, 1251–1259. - PubMed
- Bates RW; Kasinathan S. Hydroformylation in Natural Product Synthesis. Top. Curr. Chem 2013, 342, 187–223. - PubMed
- Chikkali SH; Vlugt JIVD; Reek JNH Hybrid Diphosphorus Ligands in Rhodium Catalysed Asymmetric Hydroformylation. Coord. Chem. Rev 2014, 262, 1–15.
-
- For selected reviews on the reductive carbonylation of aryl halides to form aldehydes, see: (a) Barnard CFJ Palladium-Catalyzed Carbonylation-A Reaction Come of Age. Organometallics 2008, 27, 5402–5422.
- (b) Brennführer A; Neumann H; Beller M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed 2009, 48, 4114–4133. - PubMed
-
- Siegel H; Eggersdorfer M. Ketones. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. DOI: 10.1002/14356007.a15_077. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
