Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species
- PMID: 38465216
- PMCID: PMC10920011
- DOI: 10.1016/j.jgr.2024.01.001
Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species
Abstract
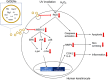
Background: Recently, plant-derived exosome-like nanoparticles (PDENs) have been isolated, and active research was focusing on understanding their properties and functions. In this study, the characteristics and molecular properties of ginseng root-derived exosome-like nanoparticles (GrDENs) were examined in terms of skin protection.
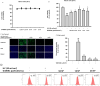
Methods: HPLC-MS protocols were used to analyze the ginsenoside contents in GrDENs. To investigate the beneficial effect of GrDENs on skin, HaCaT cells were pre-treated with GrDENs (0-2 × 109 particles/mL), and followed by UVB irradiation or H2O2 exposure. In addition, the antioxidant activity of GrDENs was measured using a fluorescence microscope or flow cytometry. Finally, molecular mechanisms were examined with immunoblotting analysis.
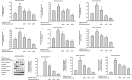
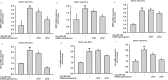
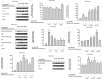
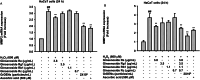
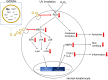
Results: GrDENs contained detectable levels of ginsenosides (Re, Rg1, Rb1, Rf, Rg2 (S), Gyp17, Rd, C-Mc1, C-O, and F2). In UVB-irradiated HaCaT cells, GrDENs protected cells from death and reduced ROS production. GrDENs downregulated the mRNA expression of proapoptotic genes, including BAX, caspase-1, -3, -6, -7, and -8 and the ratio of cleaved caspase-8, -9, and -3 in a dose-dependent manner. In addition, GrDENs reduced the mRNA levels of aging-related genes (MMP2 and 3), proinflammatory genes (COX-2 and IL-6), and cellular senescence biomarker p21, possibly by suppressing activator protein-1 signaling.
Conclusions: This study demonstrates the protective effects of GrDENs against skin damage caused by UV and oxidative stress, providing new insights into beneficial uses of ginseng. In particular, our results suggest GrDENs as a potential active ingredient in cosmeceuticals to promote skin health.
Keywords: Aging; Ginseng-derived exosome-like nanoparticles; Ginsenosides; Oxidative stress; UV irradiation.
© 2024 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Figures

Similar articles
-
Protective properties of ginsenoside Rb1 against UV-B radiation-induced oxidative stress in human dermal keratinocytes.Pharmazie. 2015 Jun;70(6):381-7. Pharmazie. 2015. PMID: 26189299
-
Stereospecificity of ginsenoside Rg2 epimers in the protective response against UV-B radiation-induced oxidative stress in human epidermal keratinocytes.J Photochem Photobiol B. 2016 Dec;165:232-239. doi: 10.1016/j.jphotobiol.2016.10.034. Epub 2016 Oct 29. J Photochem Photobiol B. 2016. PMID: 27816645
-
Ginsenoside Rk1 Prevents UVB Irradiation-Mediated Oxidative Stress, Inflammatory Response, and Collagen Degradation via the PI3K/AKT/NF-κB Pathway In Vitro and In Vivo.J Agric Food Chem. 2022 Dec 21;70(50):15804-15817. doi: 10.1021/acs.jafc.2c06377. Epub 2022 Dec 6. J Agric Food Chem. 2022. PMID: 36472249
-
Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium.Acta Pharmacol Sin. 2008 Sep;29(9):1103-8. doi: 10.1111/j.1745-7254.2008.00868.x. Acta Pharmacol Sin. 2008. PMID: 18718179 Review.
-
Advancing functional foods: a systematic analysis of plant-derived exosome-like nanoparticles and their health-promoting properties.Front Nutr. 2025 Mar 5;12:1544746. doi: 10.3389/fnut.2025.1544746. eCollection 2025. Front Nutr. 2025. PMID: 40115388 Free PMC article. Review.
Cited by
-
Alternatives of mesenchymal stem cell-derived exosomes as potential therapeutic platforms.Front Bioeng Biotechnol. 2024 Sep 9;12:1478517. doi: 10.3389/fbioe.2024.1478517. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39315312 Free PMC article. Review.
-
Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications.Int J Mol Sci. 2025 Apr 30;26(9):4277. doi: 10.3390/ijms26094277. Int J Mol Sci. 2025. PMID: 40362513 Free PMC article. Review.
-
Ginsenoside-Enriched Panax ginseng Sprouts Cultivated from Aquaponic System with a Novel Nutrient Solution Regulate LPS-Induced Inflammatory Cytokines and UVB-Induced Photoaging Responses via MAPK/AP-1 Signaling Pathways.Plants (Basel). 2025 Jun 4;14(11):1712. doi: 10.3390/plants14111712. Plants (Basel). 2025. PMID: 40508386 Free PMC article.
-
Can Panax ginseng help protect the body from the harmful effects of airborne particulate matter?J Ginseng Res. 2025 Jul;49(4):331-341. doi: 10.1016/j.jgr.2025.03.001. Epub 2025 Mar 4. J Ginseng Res. 2025. PMID: 40621080 Free PMC article. Review.
-
Recent Advances in the Isolation Strategies of Plant-Derived Exosomes and Their Therapeutic Applications.Curr Issues Mol Biol. 2025 Feb 22;47(3):144. doi: 10.3390/cimb47030144. Curr Issues Mol Biol. 2025. PMID: 40136398 Free PMC article. Review.
References
-
- Loyer X., Vion A.-C., Tedgui A., Boulanger C.M. Microvesicles as cell–cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. - PubMed
-
- Ohno S., Ishikawa A., Kuroda M. Roles of exosomes and microvesicles in disease pathogenesis. Adv Drug Deliv Rev. 2013;65:398–401. - PubMed
-
- Niu G., Jian T., Gai Y., Chen J. Microbiota and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Adv Drug Deliv Rev. 2023;196 - PubMed
-
- An Q., Hückelhoven R., Kogel K.H., van Bel A.J. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006;8:1009–1019. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

