Serum cytokine profile of neonatal broiler chickens infected with Salmonella Typhimurium
- PMID: 38465263
- PMCID: PMC10920336
- DOI: 10.3389/fphys.2024.1359722
Serum cytokine profile of neonatal broiler chickens infected with Salmonella Typhimurium
Abstract
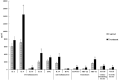
The avian immune system responds to Salmonella infection by expressing cytokines and chemokines. We hypothesized that the immune status of Salmonella Typhimurium (ST) challenged neonatal broilers would differ from the uninfected treatment. The objective of this experiment was to evaluate 12 cytokines. Day of hatch male chicks were randomly allocated into a control or ST challenged group. At day three of age, sterile diluent or 5.0 × 108 CFU of ST was given orally to each chick. Blood was obtained 24 h post challenge and serum separated for later analysis (n = 30 chicks/treatment). Significant (p ≤ 0.05) increases in pro-inflammatory cytokines-interleukin-6 (IL-6), IL-16, and IL-21; anti-inflammatory cytokines- IL-10; chemokines-regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein-1β (MIP-1β), and MIP-3α; colony stimulating factors-macrophage colony-stimulating factor (M-CSF); and growth factors-vascular endothelial growth factor (VEGF) were observed in the serum of the challenged chicks when compared to the control. No significant differences were observed in IL-2, interferon gamma (IFNγ), and IFNα. These data indicate the detection of mucosal immune responses in broiler chickens following ST infection. The heightened levels of pro-inflammatory cytokines, chemokines, and colony stimulating factors align with known inflammatory mechanisms, like the influx of immune cells. However, the elevation of IL-10 was unexpected, due to its immunoregulatory properties. Notably, the rise in VEGF levels is compelling, as it suggests the possibility of tissue repair and angiogenesis in ST infected birds.
Keywords: Salmonella Typhimurium; immune response; immunoassay; poultry; serum cytokine.
Copyright © 2024 Milby-Blackledge, Farnell, Zhao, Berghman, Laino, Muller, Byrd and Farnell.
Conflict of interest statement
Authors CL and MM were employed by the company Millipore Sigma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Akahoshi T., Sasahara T., Namai R., Matsui T., Watabe H., Kitasato H., et al. (2003). Production of macrophage inflammatory protein 3alpha (MIP-3alpha) (CCL20) and MIP-3beta (CCL19) by human peripheral blood neutrophils in response to microbial pathogens. Infect. Immun. 71, 524–526. 10.1128/IAI.71.1.524-526.2003 - DOI - PMC - PubMed
-
- Al-Khalaifah H., Al-Nasser A. (2018). Cytokines as effective elements of the avian immune system. J. Microbiol. Genet. 2018, 2574–7371. 10.29011/2574-7371.00019 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

