Engineering and application of LacI mutants with stringent expressions
- PMID: 38465475
- PMCID: PMC10926051
- DOI: 10.1111/1751-7915.14427
Engineering and application of LacI mutants with stringent expressions
Abstract
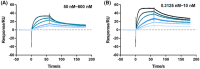
Optimal transcriptional regulatory circuits are expected to exhibit stringent control, maintaining silence in the absence of inducers while exhibiting a broad induction dynamic range upon the addition of effectors. In the Plac /LacI pair, the promoter of the lac operon in Escherichia coli is characterized by its leakiness, attributed to the moderate affinity of LacI for its operator target. In response to this limitation, the LacI regulatory protein underwent engineering to enhance its regulatory properties. The M7 mutant, carrying I79T and N246S mutations, resulted in the lac promoter displaying approximately 95% less leaky expression and a broader induction dynamic range compared to the wild-type LacI. An in-depth analysis of each mutation revealed distinct regulatory profiles. In contrast to the wild-type LacI, the M7 mutant exhibited a tighter binding to the operator sequence, as evidenced by surface plasmon resonance studies. Leveraging the capabilities of the M7 mutant, a high-value sugar biosensor was constructed. This biosensor facilitated the selection of mutant galactosidases with approximately a seven-fold improvement in specific activity for transgalactosylation. Consequently, this advancement enabled enhanced biosynthesis of galacto-oligosaccharides (GOS).
© 2024 The Authors. Microbial Biotechnology published by Applied Microbiology International and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Abraham, M.J. , Murtola, T. , Schulz, R. , Páll, S. , Smith, J.C. , Hess, B. et al. (2015) GROMACS: high performance molecular simulations through multi‐level parallelism from laptops to supercomputers. SoftwareX, 1‐2, 19–25.
-
- Bahls, M.O. , Platz, L. , Morgado, G. , Schmidt, G.W. & Panke, S. (2022) Directed evolution of biofuel‐responsive biosensors for automated optimization of branched‐chain alcohol biosynthesis. Metabolic Engineering, 69, 98–111. - PubMed
-
- Bell, C.E. & Lewis, M. (2000) A closer view of the conformation of the Lac repressor bound to operator. Nature Structural Biology, 7, 209–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Grant 2021YFC2103901/the National Key Research and Development Program of China
- 31971337/the National Natural Science Foundation of China
- 31970080/the National Natural Science Foundation of China
- 31961133016/the National Natural Science Foundation of China
- 31971382/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

